Page 116 - IJB-9-3
P. 116
International Journal of Bioprinting OMT-loaded spinal cord scaffold
Figure 1. Fabrication by 3D bioprinting and implantation of spinal cord extracellular matrix (ECM) hydrogel microfiber scaffolds equipped with
oxymatrine (OMT).
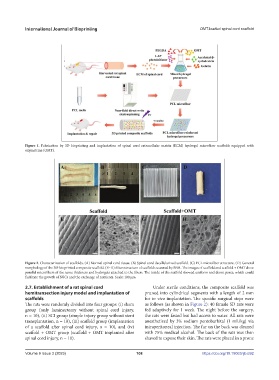
Figure 2. Characterization of scaffolds. (A) Normal spinal cord tissue. (B) Spinal cord decellularized scaffold. (C) PCL microfiber structure. (D) General
morphology of the 3D-bioprinted composite scaffold. (E–H) Microstructure of scaffolds scanned by SEM. The images of scaffold and scaffold + OMT show
parallel microfibers of the same thickness and hydrogels attached to the fibers. The inside of the scaffold showed uniform and dense pores, which could
facilitate the growth of NSCs and the exchange of nutrients. Scale: 100 μm.
2.7. Establishment of a rat spinal cord Under sterile conditions, the composite scaffold was
hemitransection injury model and implantation of pruned into cylindrical segments with a length of 2 mm
scaffolds for in vivo implantation. The specific surgical steps were
The rats were randomly divided into four groups: (i) sham as follows (as shown in Figure 2): 40 female SD rats were
group (only laminectomy without spinal cord injury, fed adaptively for 1 week. The night before the surgery,
n = 10), (ii) SCI group (simple injury group without stent the rats were fasted but had access to water. All rats were
transplantation, n = 10), (iii) scaffold group (implantation anesthetized by 3% sodium pentobarbital (1 mL/kg) via
of a scaffold after spinal cord injury, n = 10), and (iv) intraperitoneal injection. The fur on the back was cleaned
scaffold + OMT group (scaffold + OMT implanted after with 75% medical alcohol. The back of the rats was then
spinal cord injury, n = 10). shaved to expose their skin. The rats were placed in a prone
Volume 9 Issue 3 (2023) 108 https://doi.org/10.18063/ijb.692