Page 119 - IJB-9-3
P. 119
International Journal of Bioprinting OMT-loaded spinal cord scaffold
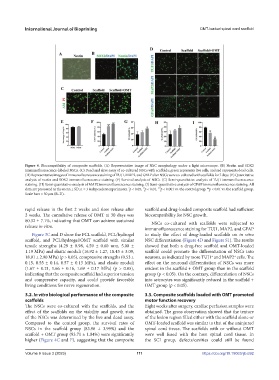
Figure 4. Biocompatibility of composite scaffolds. (A) Representative image of NSC morphology under a light microscope. (B) Nestin and SOX2
immunofluorescence-labeled NSCs. (C) Dead and alive assay of co-cultured NSCs with scaffolds; green represents live cells, and red represents dead cells.
(D) Representative images of immunofluorescence staining of TUJ1, MAP2, and GFAP after NSCs were co-cultured with scaffolds for 7 days. (E) Quantitative
analysis of nestin and SOX2 immunofluorescence staining. (F) Survival analysis of NSCs. (G) Semi-quantitative analysis of TUJ1 immunofluorescence
staining. (H) Semi-quantitative analysis of MAP2 immunofluorescence staining. (I) Semi-quantitative analysis of GFAP immunofluorescence staining. All
data are presented as the mean ± SD; n = 3 independent experiments. p < 0.05, p < 0.01, p < 0.001 vs. the control group. p < 0.01 vs. the scaffold group.
***
**
*
##
Scale bars = 50 μm (B–D).
rapid release in the first 2 weeks and slow release after scaffold and drug-loaded composite scaffold had sufficient
2 weeks. The cumulative release of OMT at 30 days was biocompatibility for NSC growth.
80.32 ± 7.1%, indicating that OMT can achieve sustained NSCs co-cultured with scaffolds were subjected to
release in vitro.
immunofluorescence staining for TUJ1, MAP2, and GFAP
Figure 3C and D show the PCL scaffold, PCL/hydrogel to study the effect of drug-loaded scaffolds on in vitro
scaffold, and PCL/hydrogel/OMT scaffold with similar NSC differentiation (Figure 4D and Figure S1). The results
tensile strengths (4.29 ± 0.98, 4.59 ± 0.60 mm, 5.00 ± showed that both a drug-free scaffold and OMT-loaded
1.19 MPa) and elastic moduli (16.92 ± 1.27, 18.43 ± 3.09, scaffold could promote the differentiation of NSCs into
18.91 ± 2.90 MPa) (p > 0.05), compressive strengths (0.53 ± neurons, as indicated by more TUJ1 and MAP2 cells. The
+
+
0.15, 0.55 ± 0.14, 0.57 ± 0.13 MPa), and elastic moduli effect on the neuronal differentiation of NSCs was more
(1.67 ± 0.21, 1.66 ± 0.16, 1.69 ± 0.17 MPa) (p > 0.05), evident in the scaffold + OMT group than in the scaffold
indicating that the composite scaffold had superior tension group (p < 0.05). On the contrary, differentiation of NSCs
and compressive capacity, and could provide favorable into astrocytes was significantly reduced in the scaffold +
living conditions for nerve regeneration. OMT group (p < 0.05).
3.2. In vitro biological performance of the composite 3.3. Composite scaffolds loaded with OMT promoted
scaffolds motor function recovery
The NSCs were co-cultured with the scaffolds, and the Eight weeks after surgery, cardiac perfusion samples were
effect of the scaffolds on the viability and growth state obtained. The gross observation showed that the texture
of the NSCs was determined by the live and dead assay. of the lesion region filled either with the scaffold alone or
Compared to the control group, the survival rates of OMT-loaded scaffold was similar to that of the uninjured
NSCs in the scaffold group (83.96 ± 3.99%) and the spinal cord tissue. The scaffolds with or without OMT
scaffold + OMT group (93.71 ± 1.04%) were significantly were well fused with the host spinal cord tissue. In
higher (Figure 4C and F), suggesting that the composite the SCI group, defects/cavities could still be found
Volume 9 Issue 3 (2023) 111 https://doi.org/10.18063/ijb.692