Page 298 - IJB-9-3
P. 298
International Journal of Bioprinting 3D bioprinting as a prospective therapeutic strategy for LSCD
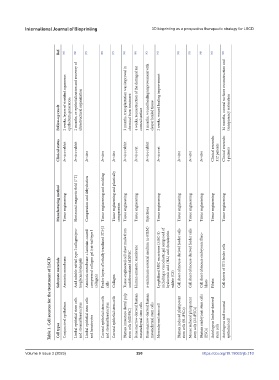
Ref. [83] [84] [85] [86] [87] [88] [89] [90] [91] [92] [93] [94] [95] [96]
2 weeks, layers of stratified squamous 2 months, re-epithelialization and recovery of ultrastructural organization 3 months, transplantation was improved in 4 weeks, reconstruction of the damaged rat 1 months, wound healing improvement with 2 weeks, wound healing improvement 14 months, corneal surfaces reconstruction and
Follow-up result epithelium generation chemical burn treatment corneal surface clearer healed tissue transparency restoration
Clinical status In vivo rabbit In vivo rabbit In vitro In vitro In vitro In vivo rabbit In vivo rat In vivo rabbit In vivo rat In vitro In vitro In vitro Clinical research: 112 patients Clinical research: 4 patients
Manufacturing method Tissue engineering Horizontal magnetic field (7 T) Compression and dehydration Tissue engineering and molding Tissue engineering and plastically compression Tissue engineering Tissue engineering Injections Tissue engineering Tissue engineering Tissue engineering Tissue engineering Tissue engineering Tissue engineering
Table 1. Cell sources for the treatment of LSCD
Substrate materials Amniotic membrane Acid soluble rat tail type I collagen/pro- teoglycan hydrogels Amniotic membrane + laminin-coated compressed collagen gel (rat-tail type I collagen) Feeder layers of lethally irradiated 3T3-J2 cells Collagen Tissue-engineered cell sheet made from undifferentiated hIDPSC Human amniotic membrane α-minimum essential medium (α-MEM) Lyophilized MSC secretome (MSC‐S) including a viscoelastic gel com
Conjunctival epithelium Limbal epithelial stem cells and stromal keratocytes Limbal epithelial stem cells Corneal epithelial stem cells and stromal keratocytes Corneal epithelial stem cells Human immature dental pulp stem cells (hIDPSC) Bone marrow-derived human mesenchymal stem cells Bone marrow-derived human mesenchymal stem cells Mesenchymal stem cell Human induced pluripotent stem cells (H-iPSCs) Mouse induced plurip
Cell types and keratocytes (ESCs) stem cells epithelial cell
Volume 9 Issue 3 (2023) 290 https://doi.org/10.18063/ijb.710