Page 351 - IJB-9-4
P. 351
International Journal of Bioprinting Bioprinting with machine learning
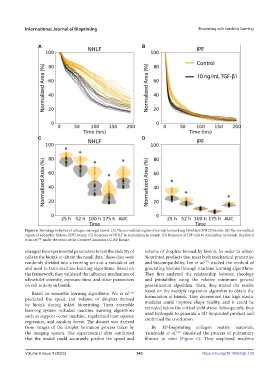
Figure 6. Shrinkage behavior of collagen microgel bioink. (A) The normalized region of normal human lung fibroblast (NHLF) bioink. (B) The normalized
region of idiopathic fibrosis (IPF) bioink. (C) Response of NHLF to stimulation in bioink. (D) Response of IPF cells to stimulation in bioink. Reprinted
from ref. under the terms of the Creative Commons CC-BY license.
[78]
changed the experimental parameters to test the viability of volume of droplets formed by bioink. In order to obtain
cells in the bioink to obtain the result data. These data were bioprinted products that meet both mechanical properties
[77]
randomly divided into a training set and a validation set and biocompatibility, Lee et al. studied the method of
and used to train machine learning algorithms. Based on generating bioinks through machine learning algorithms.
the framework, they validated the influence mechanism of They first analyzed the relationship between rheology
ultraviolet intensity, exposure time, and other parameters and printability using the relative minimum general
on cell activity in bioink. generalization algorithm. Then, they mined the results
based on the multiple regression algorithm to obtain the
Based on ensemble learning algorithms, Wu et al.
[76]
predicted the speed and volume of droplets formed formulation of bioink. They determined that high elastic
modulus could improve shape fidelity, and it could be
by bioink during inkjet bioprinting. Their ensemble extruded below the critical yield stress. Subsequently, they
learning system included machine learning algorithms used hydrogels to generate a 3D-bioprinted product and
such as support vector machine, regularized least squares confirmed the conclusion.
regression, and random forest. The dataset was derived
from images of the droplet formation process taken by By 3D-bioprinting collagen matrix materials,
the imaging system. The experimental data confirmed Yamanishi et al. simulated the process of pulmonary
[78]
that the model could accurately predict the speed and fibrosis in vitro (Figure 6). They employed machine
Volume 9 Issue 4 (2023) 343 https://doi.org/10.18063/ijb.739