Page 451 - IJB-9-4
P. 451
International Journal of Bioprinting Single-step bioink deposition and maturation of human epidermis
A B C
D E
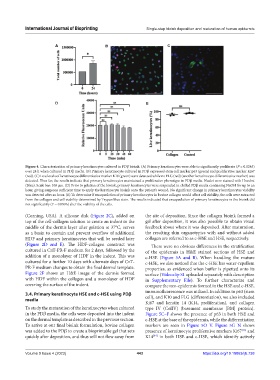
Figure 4. Characterization of primary keratinocytes cultured in PDβ bioink. (A) Primary keratinocytes were able to significantly proliferate (P = 0.0245)
over 24 h when cultured in PDβ media. (B) Primary keratinocyte cultured in PDβ expressed stem cell marker p63 (green) and proliferative marker Ki67
(red). (C) Low levels of keratinocyte differentiative marker K10 (green) were detected while no FLG (red) (another keratinocyte differentiative marker) was
detected. Thus far, the results indicate that primary keratinocytes maintained a proliferative phenotype in PDβ media. Nuclei were stained with Hoechst
(Blue). Scale bar: 100 µm. (D) Prior to gelation of the bioink, primary keratinocytes were suspended in chilled PDβ media containing NaOH for up to an
hour, giving surgeons sufficient time to apply the keratinocyte bioink onto the patient’s wound. No significant change in primary keratinocytes viability
was detected after an hour. (E) To determine if encapsulation of primary keratinocytes in bovine collagen would affect cell viability, the cells were extracted
from the collagen and cell viability determined by Trypan blue stain. The results indicated that encapsulation of primary keratinocytes in the bioink did
not significantly (P = 0.0996) alter the viability of the cells.
(Corning, USA). A silicone disk (Figure 2C), added on the site of deposition. Since the collagen bioink formed a
top of the cell-collagen solution to create an indent in the gel after deposition, it was also possible to obtain visual
middle of the dermis layer after gelation at 37°C, serves feedback about where it was deposited. After maturation,
as a basin to contain and prevent overflow of additional the resulting skin organotypics with and without added
HDF and primary keratinocytes that will be seeded later collagen are referred to as c-HSE and HSE, respectively.
(Figure 2D and E). The HDF-collagen construct was There were no obvious differences in the stratification
cultured in CnT-PR-F medium for 2 days, followed by the of the epidermis in H&E stained sections of HSE and
addition of a monolayer of HDF in the indent. This was c-HSE (Figure 5A and B). When handling the mature
cultured for a further 10 days with alternate days of CnT- c-HSE, we also noticed that the c-HSE has water-repellent
PR-F medium changes to obtain the final dermal template. properties, as evidenced when buffer is pipetted onto its
Figure 2F shows an H&E image of the dermis formed surface (Videoclip S1 uploaded separately with description
with HDF within the collagen and a monolayer of HDF in Supplementary File). To further characterize and
covering the surface of the indent. compare the neo-epidermis formed in the HSE and c-HSE,
3.4. Primary keratinocyte HSE and c-HSE using PDβ immunofluorescence was utilized. In addition to p63 (stem
media cell), and K10 and FLG (differentiation), we also included
Ki67 and keratin 14 (K14, proliferation), and collagen
To study the maturation of the keratinocytes when cultured type IV (ColIV) (basement membrane [BM] protein).
in the PDβ media, the cells were deposited into the indent Figure 5C–F shows the presence of p63 in both HSE and
on the dermal template as described in the previous section. c-HSE at the base of the epidermis, while the differentiation
To arrive at our final bioink formulation, bovine collagen markers are seen in Figure 5O–V. Figure 5G–N shows
was added to the PDβ to create a bioprintable gel that sets presence of keratinocyte proliferative markers Ki67 and
[33]
quickly after deposition, and thus will not flow away from K14 in both HSE and c-HSE, which identify actively
[34]
Volume 9 Issue 4 (2023) 443 https://doi.org/10.18063/ijb.738