Page 167 - IJB-9-5
P. 167
International Journal of Bioprinting Guide about the effects of sterilization on 3D-printed materials for medicine
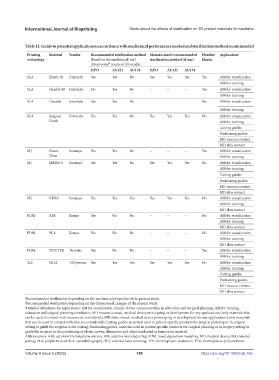
Table 12. Guide to potential applications in accordance with mechanical performance needed and sterilization method recommended
Printing Material Vendor Recommended sterilization method Manufacturer’s recommended Flexible/ Application c
technology (based on the mechanical and sterilization method (if any) Elastic
a
dimensional results of this study)
b
HPO AU121 AU134 HPO AU121 AU134
SLA Elastic 50 Formlabs Yes Yes No Yes Yes Yes Yes AM for visualization
AM for training
SLA Flexible 80 Formlabs No Yes No – – – Yes AM for visualization
AM for training
SLA Durable Formlabs Yes Yes No – – – No AM for visualization
AM for training
SLA Surgical Formlabs Yes Yes No Yes Yes Yes No AM for visualization
Guide AM for training
Cutting guides
Positioning guides
MD mucous contact
MD skin contact
MJ Elastic Stratasys Yes Yes No – – – Yes AM for visualization
Clear AM for training
MJ MED610 Stratasys Yes Yes No Yes Yes Yes No AM for visualization
AM for training
Cutting guides
Positioning guides
MD mucous contact
MD skin contact
MJ VERO Stratasys Yes Yes Yes Yes Yes Yes No AM for visualization
AM for training
MD skin contact
FDM ABS Kimya Yes No No – – – No AM for visualization
AM for training
MD skin contact
FDM PLA Kimya Yes No No – – – No AM for visualization
AM for training
MD skin contact
FDM TPU/TPE Recreus Yes No No – – – Yes AM for visualization
AM for training
SLS PA12 3D Systems Yes Yes Yes Yes Yes Yes No AM for visualization
AM for training
Cutting guides
Positioning guides
MD mucous contact
MD skin contact
a Recommended sterilization depending on the mechanical properties of the present study.
b Recommended sterilization depending on the dimensional changes of the present study.
c Detailed definitions for applications: AM for visualization, family–doctor communication in education and surgical planning; AM for training,
education and surgical planning simulation; MD mucous contact, medical device prototyping or development for any applications (only materials that
can be used in contact with mucous are considered); MD skin contact, medical device prototyping or development for any applications (only materials
that can be used in contact with skin are considered); Cutting guides, materials used in patient-specific products for surgical planning or in surgery
setting to guide the surgeon in the cutting; Positioning guides, materials used in patient-specific products for surgical planning or in surgery setting to
guide the surgeon in the positioning of plates, screws, distractors and other implanted or temporary material.
Abbreviations: ABS, acrylonitrile butadiene styrene; AM, additive manufacturing; FDM, fused deposition modeling; MD, medical device; MJ, material
jetting; PLA, polylactic acid; SLA, stereolithography; SLS, selective laser sintering; TPE, thermoplastic elastomer; TPU, thermoplastic polyurethane.
Volume 9 Issue 5 (2023) 159 https://doi.org/10.18063/ijb.756