Page 258 - IJB-9-5
P. 258
International Journal of Bioprinting Antheraea pernyi silk fibroin bioinks for DLP 3D printing
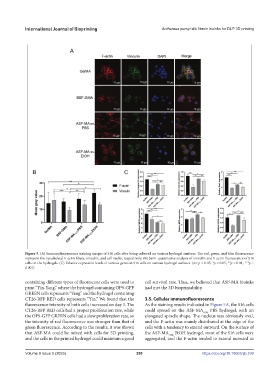
Figure 5. (A) Immunofluorescence staining images of S16 cells after being cultured on various hydrogel surfaces. The red, green, and blue fluorescence
represent the cytoskeletal F-actin fibers, vinculin, and cell nuclei, respectively. (B) Semi-quantitative analysis of vinculin and F-actin fluorescence of S16
cells on the hydrogels. (C) Relative expression levels of various genes in S16 cells on various hydrogel surfaces. (ns: p > 0.05, *p < 0.05, **p < 0.01, ***p <
0.001)
containing different types of fluorescent cells were used to cell survival rate. Thus, we believed that ASF-MA bioinks
print “Yin-Yang,” where the hydrogel containing OP9-GFP had met the 3D bioprintability.
GREEN cells represents “Yang” and the hydrogel containing
CT26-RFP RED cells represents “Yin.” We found that the 3.5. Cellular immunofluorescence
fluorescence intensity of both cells increased on day 3. The As the staining results indicated in Figure 5A, the S16 cells
CT26-RFP RED cells had a proper proliferation rate, while could spread on the ASF-MA PBS hydrogel, with an
10%
the OP9-GFP GREEN cells had a slow proliferation rate, so elongated spindle shape. The nucleus was obviously oval,
the intensity of red fluorescence was stronger than that of and the F-actin was mainly distributed at the edge of the
green fluorescence. According to the results, it was shown cells with a tendency to extend outward. On the surface of
that ASF-MA could be mixed with cells for 3D printing, the ASF-MA 10% EtOH hydrogel, most of the S16 cells were
and the cells in the printed hydrogel could maintain a good aggregated, and the F-actin tended to extend outward as
Volume 9 Issue 5 (2023) 250 https://doi.org/10.18063/ijb.760