Page 315 - IJB-9-5
P. 315
International Journal of Bioprinting Scaffold for engineering enthesis organ
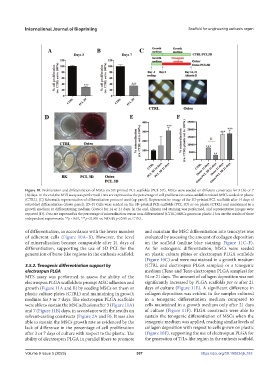
Figure 10. Proliferation and differentiation of MSCs on 3D-printed PCL scaffolds (PCL 3D). MSCs were seeded on different constructs for 3 (A) or 7
(B) days. In the end, the MTS assay was performed. Data are expressed as the percentage of cell proliferation versus undifferentiated MSCs seeded on plastic
(CTRL). (C) Schematic representation of differentiation protocol used (up panel). Representative image of the 3D-printed PCL scaffolds after 14 days of
osteoblast differentiation (down panel). (D–F) Cells were seeded on the 3D-printed PCL scaffolds (PCL 3D) or on plastic (CTRL) and maintained in a
growth medium or differentiating medium (Osteo) for 14 or 21 days. In the end, alizarin red staining was performed, and representative images were
reported (D). Data are expressed as the percentage of mineralization versus non-differentiated (CTRL) MSCs grown on plastic. Data are the results of three
independent experiments. **p < 0.01, ***p < 0.001 vs. ND; §§ p < 0.01 vs. CTRL.
of differentiation, in accordance with the lower number and maintain the MSC differentiation into tenocytes was
of adherent cells (Figure 10A–B). However, the level evaluated by assessing the amount of collagen deposition
of mineralization became comparable after 21 days of on the scaffold (aniline blue staining; Figure 11C–F).
differentiation, supporting the use of 3D PCL for the As for osteogenic differentiation, MSCs were seeded
generation of bone-like regions in the enthesis scaffold. on plastic culture plates or electrospun PLGA scaffolds
(Figure 10C) and were maintained in a growth medium
3.5.2. Tenogenic differentiation support by (CTRL and electrospun PLGA samples) or a tenogenic
electrospun PLGA medium (Teno and Teno electrospun PLGA samples) for
MTS assay was performed to assess the ability of the 14 or 21 days. The amount of collagen deposition was not
electrospun PLGA scaffolds to prompt MSC adhesion and significantly increased by PLGA scaffolds per se after 21
growth (Figure 11A and B) by seeding MSCs on them or days of culture (Figure 11E). A significant difference in
plastic culture plates (CTRL) and maintaining in growth collagen deposition was evident in the samples cultured
medium for 3 or 7 days. The electrospun PLGA scaffolds in a tenogenic differentiation medium compared to
were able to sustain the MSC adhesion after 3 (Figure 11A) cells maintained in a growth medium only after 21 days
and 7 (Figure 11B) days, in accordance with the results on of culture (Figure 11F). PLGA constructs were able to
solvent-casting constructs (Figure 2A and B). It was also sustain the tenogenic differentiation of MSCs when the
able to sustain the MSCs growth rate as evidenced by the tenogenic medium was applied, reaching similar levels of
lack of difference in the percentage of cell proliferation collagen deposition with respect to cells grown on plastic
after 3 or 7 days of culture with respect to the plastic. The (Figure 10F), supporting the use of electrospun PLGA for
ability of electrospun PLGA in parallel fibers to promote the generation of T/Ls-like region in the enthesis scaffold.
Volume 9 Issue 5 (2023) 307 https://doi.org/10.18063/ijb.763