Page 318 - IJB-9-5
P. 318
International Journal of Bioprinting Scaffold for engineering enthesis organ
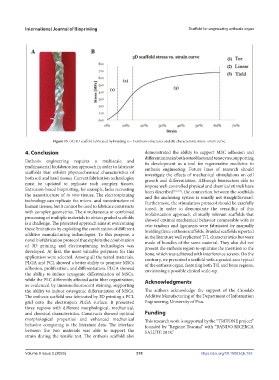
Figure 13. (A) 3D scaffold fabricated by braiding n = 3 enthesis structures and (B) characteristic stress–strain curve.
4. Conclusion demonstrated the ability to support MSC adhesion and
differentiation in both osteoblasts and tenocytes, supporting
Enthesis engineering requires a multiscale and its development as a tool for regenerative medicine in
multimaterial biofabrication approach in order to fabricate enthesis engineering. Future lines of research should
scaffolds that exhibit physicochemical characteristics of investigate the effects of mechanical stimulations on cell
both soft and hard tissues. Current fabrication technologies growth and differentiation. Although bioreactors able to
must be updated to replicate such complex tissues. impose well-controlled physical and chemical stimuli have
Extrusion-based bioprinting, for example, lacks recreating been described [51–53] , the connection between the scaffolds
the nanostructure of in vivo tissues. The electrospinning and the anchoring system is usually not straightforward.
technology can replicate the micro- and nanostructure of Furthermore, the stimulation protocol should be carefully
human tissues, but it cannot be used to fabricate constructs tuned. In order to demonstrate the versatility of this
with complex geometries. The simultaneous or combined biofabrication approach, clinically relevant scaffolds that
processing of multiple materials to obtain graded scaffolds showed optimal mechanical behavior comparable with in
is a challenge. The presented approach aims at overcoming vivo tendons and ligaments were fabricated by manually
these limitations by exploiting the combination of different braiding three enthesis scaffolds. Braided scaffolds reported
additive manufacturing technologies. To this purpose, a in the literature well replicated T/L characteristics but were
novel biofabrication protocol that exploits the combination made of bundles of the same material. They also did not
of 3D printing and electrospinning technologies was present the enthesis region to optimize the insertion to the
developed. At first, the most valuable polymers for this bone, which was achieved with interference screws. On the
application were selected. Among all the tested materials, contrary, we presented a scaffold with a graded area typical
PLGA and PCL showed a better ability to promote MSCs of the enthesis organ, featuring both T/L and bone regions,
adhesion, proliferation, and differentiation. PLGA showed envisioning a possible clinical scale-up.
the ability to induce tenogenic differentiation of MSCs,
while the PLC differently affected actin fiber organization, Acknowledgments
as evidenced by immunofluorescent staining, supporting
the ability to induce osteogenic differentiation of MSCs. The authors acknowledge the support of the Crosslab
The enthesis scaffold was fabricated by 3D printing a PCL Additive Manufacturing of the Department of Information
grid onto the electrospun PLGA surface. It presented Engineering, University of Pisa.
three regions with different morphological, mechanical,
and chemical characteristics. Constructs showed optimal Funding
morphological properties and enhanced mechanical This research work is supported by the “TRITONE project”
behavior comparing to the literature data. The interface founded by “Regione Toscana” with “BANDO RICERCA
between the two materials was able to support the SALUTE 2018.”
strain during the tensile test. The enthesis scaffold also
Volume 9 Issue 5 (2023) 310 https://doi.org/10.18063/ijb.763