Page 491 - IJB-9-5
P. 491
International Journal of Bioprinting CECM-GelMA bioinks of DLP 3D printing for corneal engineering
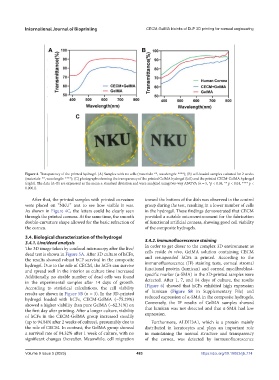
Figure 4. Transparency of the printed hydrogel. (A) Samples with no cells (materials: **, wavelength: ****); (B) cell-loaded samples cultured for 2 weeks
(materials: **, wavelength: ****); (C) photographs showing the transparency of the printed GelMA hydrogel (left) and the printed CECM-GelMA hydrogel
(right). The data (A–B) are expressed as the mean ± standard deviation and were analyzed using two-way ANOVA (n = 5, *p < 0.05, ** p < 0.01, **** p <
0.0001).
After that, the printed samples with printed curvature toward the bottom of the dish was observed in the control
were placed on “NKU” text to see how visible it was. group during the test, resulting in a lower number of cells
As shown in Figure 4C, the letters could be clearly seen in the hydrogel. These findings demonstrated that CECM
through the printed corneas. At the same time, the smooth provided a suitable microenvironment for the fabrication
double-curvature shape allowed for the basic refraction of of functional artificial corneas, showing good cell viability
the cornea. of the composite hydrogels.
3.4. Biological characterization of the hydrogel
3.4.1. Live/dead analysis 3.4.2. Immunofluorescence staining
The 3D image taken by confocal microscopy after the live/ In order to get closer to the complex 3D environment as
dead test is shown in Figure 5A. After 3D culture of hCFs, cells reside in vivo, GelMA solution containing CECM
the results showed robust hCF survival in the composite and resuspended hCFs is printed. According to the
hydrogel. Due to the role of CECM, the hCFs can survive immunofluorescence (IF) staining tests, corneal stromal
and spread well in the interior as culture time increased functional protein (lumican) and corneal myofibroblast-
Additionally, no sizable number of dead cells was found specific marker (α-SMA) in the 3D-printed samples were
in the experimental samples after 14 days of growth. detected. After 1, 7, and 14 days of culture, the results
According to statistical calculations, the cell viability (Figure 6) showed that hCFs exhibited high expression
results are shown in Figure 5B (n = 3). In the 3D-printed of lumican (Figure S8 in Supplementary File) and
hydrogel loaded with hCFs, CECM-GelMA (~75.29%) reduced expression of α-SMA in the composite hydrogels.
showed a higher viability than pure GelMA (~62.31%) on Conversely, the IF results of GelMA samples showed
the first day after printing. After a longer culture, viability that lumican was not detected and that α-SMA had low
of hCFs in the CECM-GelMA group increased steadily expression.
(up to 94.84% after 2 weeks of culture), presumably due to Furthermore, ALDH3A1, which is a protein mainly
the role of CECM. In contrast, the GelMA group showed distributed in keratocytes and plays an important role
a survival rate of 84.32% after 1 week of culture, with no in maintaining the normal structure and transparency
significant changes thereafter. Meanwhile, cell migration of the cornea, was detected by immunofluorescence
Volume 9 Issue 5 (2023) 483 https://doi.org/10.18063/ijb.774