Page 494 - IJB-9-5
P. 494
International Journal of Bioprinting CECM-GelMA bioinks of DLP 3D printing for corneal engineering
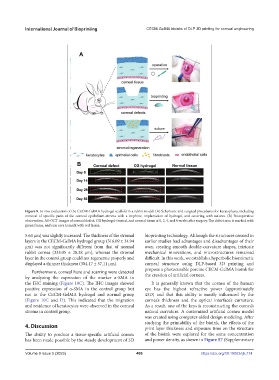
Figure 8. In vivo evaluation of the CECM-GelMA hydrogel scaffold in a rabbit model. (A) Schematic and surgical procedures for keratoplasty, including
removal of specific parts of the corneal epithelium-stroma with a trephine, implantation of hydrogel, and covering with sutures. (B) Postoperative
observation. AS-OCT images of corneal defect, CG hydrogel-treated, and normal tissue at 0, 2, 4, and 8 weeks after surgery. The defect area is marked with
green frame, and scar area is mark with red frame.
5.63 μm) was slightly increased. The thickness of the stromal bioprinting technology. Although the structures created in
layers in the CECM-GelMA hydrogel group (316.09 ± 34.94 earlier studies had advantages and disadvantages of their
μm) was not significantly different from that of normal own, creating smooth double-curvature shapes, intricate
rabbit cornea (333.05 ± 28.38 μm), whereas the stromal mechanical innovations, and microstructures remained
layer in the control group could not regenerate properly and difficult. In this work, we establish a hyperbolic biomimetic
displayed a thinner thickness (304.17 ± 57.11 μm). corneal structure using DLP-based 3D printing and
Furthermore, corneal haze and scarring were detected propose a photocurable porcine CECM-GelMA bioink for
by analyzing the expression of the marker α-SMA in the creation of artificial corneas.
the IHC staining (Figure 10C). The IHC images showed It is generally known that the cornea of the human
positive expression of α-SMA in the control group but eye has the highest refractive power (approximately
not in the CECM-GelMA hydrogel and normal group 43D) and that this ability is mostly influenced by the
(Figure 10C and D). This indicated that the migration cornea’s thickness and the optical interface’s curvature.
and residence of keratocytes were observed in the corneal As a result, one of the keys is reconstructing the cornea’s
stroma in control group. natural curvature. A customized artificial cornea model
was created using computer-aided design modeling. After
studying the printability of the bioink, the effects of the
4. Discussion print layer thickness and exposure time on the structure
The ability to produce a tissue-specific artificial cornea of the bioink were explored for the same concentration
has been made possible by the steady development of 3D and power density, as shown in Figure S7 (Supplementary
Volume 9 Issue 5 (2023) 486 https://doi.org/10.18063/ijb.774