Page 506 - IJB-9-5
P. 506
International Journal of Bioprinting Implantation of composites for cartilage repair
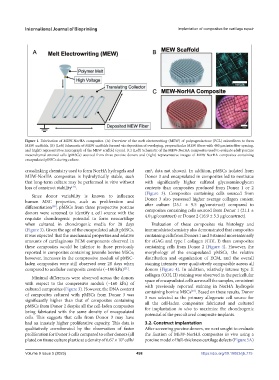
Figure 1. Fabrication of MEW-NorHA composites. (A) Overview of the melt electrowriting (MEW) of polycaprolactone (PCL) microfibers to form
MEW scaffolds. (B) (Left) Schematic of MEW scaffolds formed via deposition of overlaying, perpendicular MEW fibers with 400 μm interfiber spacing,
and (right) representative micrograph of the MEW scaffold (cyan). (C) (Left) Schematic of the MEW-NorHA composites used to evaluate adult porcine
mesenchymal stromal cells (pMSCs) sourced from three porcine donors and (right) representative images of MEW-NorHA composites containing
encapsulated pMSCs during culture.
crosslinking chemistry used to form NorHA hydrogels and cm , data not shown). In addition, pMSCs isolated from
2
MEW-NorHA composites is hydrolytically stable, such Donor 3 and encapsulated in composites led to neotissue
that long-term culture may be performed in vitro without with significantly higher sulfated glycosaminoglycan
loss of construct stability . contents than composites produced from Donor 1 or 2
[37]
(Figure 3). Composites containing cells sourced from
Since donor variability is known to influence
human MSC properties, such as proliferation and Donor 3 also possessed higher average collagen content
after culture (25.1 ± 9.5 μg/construct) compared to
differentiation , pMSCs from three prospective porcine composites containing cells sourced from Donor 1 (21.1 ±
[38]
donors were screened to identify a cell source with the 4.0 μg/construct) or Donor 2 (16.9 ± 3.3 μg/construct).
requisite chondrogenic potential to form neocartilage
when cultured in chondrogenic media for 28 days Evaluation of these composites via histology and
(Figure 3). Given the age of the encapsulated adult pMSCs, immunohistochemistry also demonstrated that composites
it was expected that the mechanical properties and relative containing cells from Donors 1 and 3 stained more intensely
amounts of cartilaginous ECM components observed in for sGAG and type I collagen (COL I) than composites
these composites would be inferior to those previously containing cells from Donor 2 (Figure 4). However, the
reported in composites containing juvenile bovine MSCs; morphology of the encapsulated pMSCs, the relative
however, increases in the compressive moduli of pMSC- distribution and organization of ECM, and the overall
laden composites were still observed over 28 days when staining intensity were qualitatively comparable across all
compared to acellular composite controls (~100 kPa) . donors (Figure 4). In addition, relatively intense type II
[21]
collagen (COL II) staining was observed in the pericellular
Minimal differences were observed across the donors
with respect to the compressive moduli (~160 kPa) of space of encapsulated cells across all the samples, consistent
with previously reported staining in NorHA hydrogels
cultured composites (Figure 3). However, the DNA content containing bovine MSCs . Based on these results, Donor
[23]
of composites cultured with pMSCs from Donor 3 was 3 was selected as the primary allogeneic cell source for
significantly higher than that of composites containing all the cell-laden composites fabricated and cultured
pMSCs from Donor 2 despite all the cell-laden composites for implantation in vivo to maximize the chondrogenic
being fabricated with the same density of encapsulated potential of the precultured composite implants.
cells. This suggests that cells from Donor 3 may have
had an innately higher proliferative capacity. This data is 3.2. Construct implantation
qualitatively corroborated by the observation of faster After screening porcine donors, we next sought to evaluate
proliferation for Donor 3 cells compared to other donors (all the fixation of MEW-NorHA composites in vivo using a
plated on tissue culture plastic at a density of 6.67 × 10 cells/ porcine model of full-thickness cartilage defects (Figure 5A)
3
Volume 9 Issue 5 (2023) 498 https://doi.org/10.18063/ijb.775