Page 85 - IJB-9-5
P. 85
International Journal of Bioprinting Biocompatible BSA-GMA and TPP of 3D hydrogels with free radical type I photoinitiator
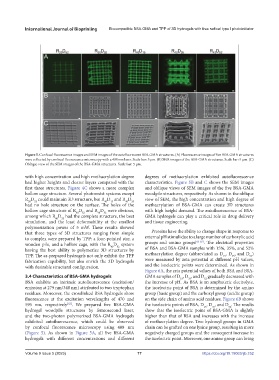
Figure 5. Confocal fluorescence images and SEM images of the autofluorescent BSA-GMA structures. (A) Fluorescence images of five BSA-GMA structures
were collected by confocal fluorescence microscopy with a 488 nm laser. Scale bar: 5 μm. (B) SEM images of the BSA-GMA structures. Scale bar: 5 μm. (C)
Oblique view of the SEM images of the BSA-GMA structures. Scale bar: 5 μm.
with high concentration and high methacrylation degree degrees of methacrylation exhibited autofluorescence
had higher heights and clearer layers compared with the characteristics. Figure 5B and C shows the SEM images
first three structures. Figure 4C shows a more complex and oblique views of SEM images of the five BSA-GMA
hollow cage structure. Several photoresist systems except woodpile structures, respectively. As shown in the oblique
R D could maintain 3D structure, but R D and R D view of SEM, the high concentration and high degree of
20
40
15
52
35
40
had no hole structure on the surface. The holes of the methacrylation of BSA-GMA can create 3D structures
hollow cage structure of R D and R D were obvious, with high height demand. The autofluorescence of BSA-
30
40
52
52
among which R D had the complete structure, the best GMA hydrogels can play a critical role in drug delivery
52
40
simulation, and the least deformability at the smallest and tissue engineering.
polymerization power of 5 mW. These results showed
that three types of 3D structures ranging from simple Proteins have the ability to change shape in response to
to complex were prepared by TPP: a four-pointed star, a external pH stimuli due to a large number of carboxylic acid
[63-65]
wooden pile, and a hollow cage, with the R D system groups and amino groups . The electrical properties
52
40
having the best ability to polymerize 3D structures by of BSA and BSA-GMA samples with 15%, 35%, and 52%
TPP. The as-prepared hydrogels not only exhibit the TPP methacrylation degree (abbreviated as D , D , and D )
52
15
35
fabrication capability, but also enrich the 3D hydrogels were measured by zeta potential at different pH values,
with desirable structural configuration. and the isoelectric points were determined. As shown in
Figure 6A, the zeta potential values of both BSA and BSA-
3.4 Characteristics of BSA-GMA hydrogels GMA samples of D , D , and D gradually decreased with
52
15
35
BSA exhibits an intrinsic autofluorescence (excitation/ the increase of pH. As BSA is an amphoteric electrolyte,
emission at 279 nm/348 nm) attributed to two tryptophan the isoelectric point of BSA is determined by the amino
residues. Moreover, the crosslinked BSA hydrogels show group (basic group) and the carboxyl group (acidic group)
fluorescence at the excitation wavelengths of 470 and on the side chain of amino acid residues. Figure 6B shows
595 nm, respectively . We prepared five BSA-GMA the isoelectric points of BSA, D , D , and D . The results
[62]
15
35
52
hydrogel woodpile structures by femtosecond laser, show that the isoelectric point of BSA-GMA is slightly
and the two-photon polymerized BSA-GMA hydrogels higher than that of BSA and increases with the increase
exhibited autofluorescence, which could be observed of methacrylation degree. Two hydroxyl groups in GMA
by confocal fluorescence microscopy using 488 nm chain can be grafted on one lysine group, resulting in more
(Figure 5). As shown in Figure 5A, all five BSA-GMA negatively charged groups and the consequent increase in
hydrogels with different concentrations and different the isoelectric point. Moreover, one amino group can bring
Volume 9 Issue 5 (2023) 77 https://doi.org/10.18063/ijb.752