Page 300 - IJB-9-6
P. 300
International Journal of Bioprinting Progress in bioprinted ear reconstruction
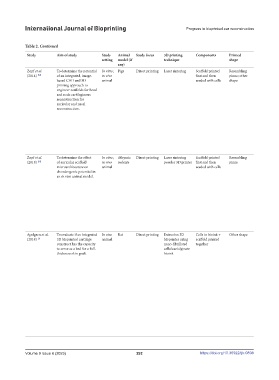
Table 2. Continued
Study Aim of study Study Animal Study focus 3D printing Components Printed Printed Cell nature/type Notable post- Assessment Findings Limitations and suggested
setting model (if technique shape material printing of success/ improvements
any) modifications integration
Zopf et al. To determine the potential In vitro; Pigs Direct printing Laser sintering Scaffold printed Resembling PCL Chondrocytes Chondrocytes Histopathology • A novel image-based CAD/CAM 3D printing Implantation into the pigs
(2014) [52] of an integrated, image- in vivo first and then pinna; other were isolated from were seeded into process in the production of bioresorbable PCL was only to demonstrate
based CAD and 3D animal seeded with cells shape harvested porcine the auricular PCL scaffolds with a defined porous architecture for appearance, so long-term in
printing approach to auricular cartilage scaffolds using a cartilaginous frameworks was introduced. The vivo performance was not
engineer scaffolds for head type I collagen gel. technique utilized 3D printing for auricular studied.
and neck cartilaginous The cell suspension and nasal scaffold production and control of
reconstruction for was pipetted into the pore architecture.
auricular and nasal the PCL scaffolds, • The surgical implantation of scaffolds was
reconstruction. and the constructs performed with ease, and scaffold porosity
were placed in an allowed for the versatility and ease of suture
incubator (37°C, placement. The PCL material maintained
5% CO ) for 30 excellent foundational support and appearance
2
minutes for gelation when implanted in subcutaneous tissue.
to occur. • The PCL scaffolds all demonstrated
histologically native-appearing cartilage
growth. However, the scaffolds had not
reached complete confluent cartilage coverage
after two months in vitro.
Zopf et al. To determine the effect In vitro; Athymic Direct printing Laser sintering Scaffold printed Resembling PCL Porcine auricular Chondrocytes Histopathology • Auricular constructs with two micropore • Short time in vivo
(2018) [54] of auricular scaffold in vivo rodents powder 3D printer first and then pinna cartilage seeded into the architectures were rapidly manufactured with (4 weeks)
microarchitecture on animal seeded with cells chondrocytes auricular PCL high-fidelity anatomic appearance. • No mechanical testing of
chondrogenic potential in scaffolds using a • Subcutaneous implantation of the scaffolds scaffold
an in vivo animal model. type I collagen/ resulted in excellent external appearance of • Study size unstated
HA composite gel. both anterior and posterior auricular surfaces.
Seeded constructs • Comparing two different microporous
were cultured in scaffold architectures demonstrated the
sterile, dynamic importance of scaffold design in optimizing
conditions with auricular cartilage growth.
incubation at 37°C, • Results suggest that a defined arrangement of
5% CO . After 4 spherical pores yielded more robust auricular
2
weeks of in vitro tissue growth.
culture, they were • The creation of spherical micropores within
implanted into the scaffold architecture appears to impart
rodents. greater chondrogenicity to the bioscaffold.
Apelgren et al. To evaluate if an integrated In vivo Rat Direct printing Extrusion 3D Cells in bioink + Other shape Bioink Human bone After printing, Histopathology • 3D-bioprinted cartilage that has been allowed • Since rodents heal by
(2018) [3] 3D bioprinted cartilage animal bioprinter using scaffold printed marrow–derived constructs were to integrate in vivo is a sufficient base for a withering, they may
construct has the capacity nano-fibrillated together mesenchymal stem crosslinked with full-thickness skin graft. not be the ideal model
to serve as a bed for a full- cellulose/alginate cells & human 100 mM CaCl for • Transplanted skin survived subcutaneously in for studying the human
2
thickness skin graft. bioink nasal chondrocytes 5 minutes in 37°C. all the 20 surviving animals. healing process in all
The constructs • No necrosis was observed. There were no aspects.
were washed macroscopic signs of complications. • Moreover, rodents’
with a balanced • However, lymphocytic infiltration was skin lacks apocrine
salt solution. observed as a sign of inflammatory activity. sweat glands and is
The printed • The majority of the chondrocytes did not proportionally much
constructs were survive (speculated to be due to diffusion thinner than humans’
then immediately limit). and has an additional
implanted muscle layer that makes
subcutaneously into the comparisons difficult.
the mice. • Short study time. The
long-term outcome of
shape stability, elastic
features, and tissue
integrity are crucial
factors that have to be
addressed in further
studies.
Volume 9 Issue 6 (2023) 292 https://doi.org/10.36922/ijb.0898