Page 389 - IJB-9-6
P. 389
International Journal of Bioprinting Biomimetic biofabrication of tumors volume
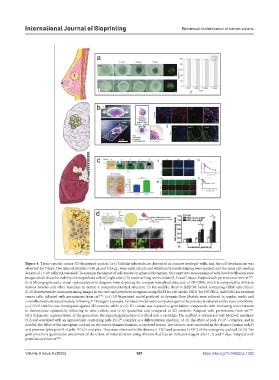
Figure 4. Tissue-specific cancer 3D-bioprinted models. (a-i) Cellular spheroids are deposited in concave hydrogel wells, and the cell development was
observed for 7 days. Two sizes of droplets, 0.48 μL and 0.23 μL, were used; calcein and ethidium bromide staining were applied, and the same cell-seeding
6
density of 1 × 10 cells/mL was used. To examine the impact of cell density on spheroid formation, the constructs were examined with live/dead fluorescence
[68]
images which show the viability of encapsulated cells (ii) right after (1 h) and over long-term culture (3, 5, and 7 days). Adapted with permissions from ref. .
(b-i) Micrographs and a visual representation in diagram form depicting the compartmentalized structure of 3D GBM, which is composed by different
various bioinks and other materials to mimic a compartmentalized structure. In the middle, there is BdECM bioink containing GBM cells (blue).
(b-ii) Representative immunostaining images in the core and peripherical regions using DAPI for cell nuclei, CD31 for HUVECs, and SOX2 for resistant
[78]
cancer cells. Adapted with permissions from ref. . (c-i) 3D-bioprinted model perfused in dynamic flow. Models were cultured in regular media and
controlled with untreated models, following XTT reagent exposure. Perfused models were compared against the constructs cultured under static conditions.
(c-ii) Cell viability was investigated against 2D controls, while (c-iii) 3D culture was exposed to gemcitabine compounds with increasing concentration
to demonstrate cytotoxicity following in vitro culture and (c-iv) quantified and compared to 2D controls. Adapted with permissions from ref. .
[83]
(d-i) Schematic representation of the procedure: the alginate/gelatin/Saos-2 is filled into a cartridge. The scaffold is submersed into McCoy’s medium/
+
FCS and overlayed with an agarose layer containing poly-P∙Ca² -complex as a differentiation medium. (d-ii) The effect of poly-P∙Ca²+-complex, and in
parallel, the effect of the osteogenic cocktail on the extent of mineralization, is reported herein. The cultures were incubated in the absence (minus polyP)
and presence (plus polyP) of poly-P∙Ca²+-complex. They were observed in the absence (- OC) and presence (+ OC) of the osteogenic cocktail. (d-iii) The
graft presents a quantitative assessment of the extent of mineralization using Alizarin Red S as an indicator reagent after 1, 5, and 7 days. Adapted with
permissions from ref. .
[98]
Volume 9 Issue 6 (2023) 381 https://doi.org/10.36922/ijb.1022