Page 40 - IJB-9-6
P. 40
International Journal of Bioprinting Transdermal delivery of printed cisplatin
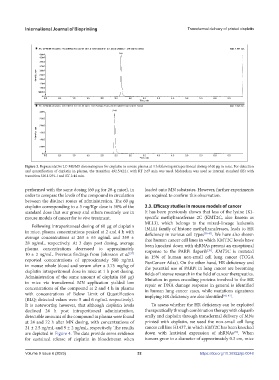
Figure 3. Representative LC-MS/MS chromatogram for cisplatin in mouse plasma at 4 h following intraperitoneal dosing of 60 μg in mice. For detection
and quantification of cisplatin in plasma, the transition 492.5/422.1 with RT 2.67 min was used. Midazolam was used as internal standard (IS) with
transition 326.1/291.1 and RT 2.44 min.
performed with the same dosing (60 μg for 20-g mice), in loaded onto MN substrates. However, further experiments
order to compare the levels of the compound in circulation are required to confirm this observation.
between the distinct routes of administration. The 60 μg
cisplatin corresponding to a 3 mg/Kgr dose is 50% of the 3.3. Efficacy studies in mouse models of cancer
standard dose that our group and others routinely use in It has been previously shown that loss of the lysine (K)‐
mouse models of cancer for in vivo treatment. specific methyltransferase 2C (KMT2C, also known as
MLL3), which belongs to the mixed‐lineage leukemia
Following intraperitoneal dosing of 60 μg of cisplatin (MLL) family of histone methyltransferases, leads to HR
in mice, plasma concentrations peaked at 2 and 4 h with deficiency in various cell types [38-40] . We have also shown
average concentrations at 263 ± 63 ng/mL and 359 ± that human cancer cell lines in which KMT2C levels have
28 ng/mL, respectively. At 3 days post dosing, average been knocked down with shRNAs present an exceptional
plasma concentrations decreased to approximately response to the PARPi ilaparib . KMT2C is mutated
[38]
10 ± 2 ng/mL. Previous findings from Johnsson et al. in 15% of human non-small cell lung cancer (TCGA
[37]
reported concentrations of approximately 500 ng/mL PanCancer Atlas). On the other hand, HR deficiency and
in mouse whole blood and serum after a 3.75 mg/kg of the potential use of PARPi in lung cancer are becoming
cisplatin intraperitoneal dose in mice at 1 h post dosing. fields of intense research in the field of cancer therapeutics.
Administration of the same amount of cisplatin (60 μg) Mutation in genes encoding proteins involved in the HR
in mice via transdermal MN application yielded low repair or DNA damage response in general is identified
concentrations of the compound at 2 and 4 h in plasma in human lung cancer cases, while mutations signatures
with concentrations of Below Limit of Quantification implying HR deficiency are also identified [41-43] .
(BLQ; detected values were 5 and 6 ng/mL respectively).
It is noteworthy, however, that although cisplatin levels To assess whether the HR deficiency can be exploited
declined 24 h post intraperitoneal administration, therapeutically through combination therapy with olaparib
detectable amounts of the compound in plasma were found orally and cisplatin through transdermal delivery of MNs
at 24 and 72 h after MN dosing, with concentrations of printed with cisplatin, we used the non-small cell lung
21 ± 2.5 ng/mL and 9 ± 2 ng/mL, respectively. The results cancer cell line H1437, in which KMT2C has been knocked
[38]
are depicted in Figure 4. The data provide some evidence down with lentiviral expression of shRNAs . When
for sustained release of cisplatin in bloodstream when tumors grew to a diameter of approximately 0.3 cm, mice
Volume 9 Issue 6 (2023) 32 https://doi.org/10.36922/ijb.0048