Page 41 - IJB-9-6
P. 41
International Journal of Bioprinting Transdermal delivery of printed cisplatin
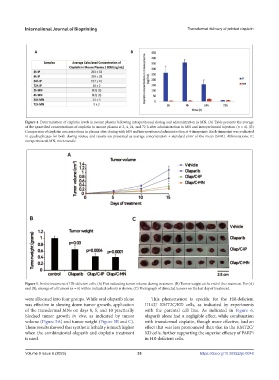
Figure 4. Determination of cisplatin levels in mouse plasma following intraperitoneal dosing and administration in MN. (A) Table presents the average
of the quantified concentrations of cisplatin in mouse plasma at 2, 4, 24, and 72 h after administration in MN and intraperitoneal injection (n = 4). (B)
Comparison of cisplatin concentrations in plasma after dosing with MN and intraperitoneal administration at 4 timepoints. Each timepoint was evaluated
in quadruplicates for both dosing routes, and results are presented as average concentration ± standard error of the mean (SEM). Abbreviations: IP,
intraperitoneal; MN, microneedle.
Figure 5. In vivo treatment of HR-deficient cells. (A) Plot indicating tumor volume during treatment. (B) Tumor weight at the end of the treatment. For (A)
and (B), average of all tumors (n = 8) within indicated cohorts is shown. (C) Photograph of dissected tumors on the last day of treatment.
were allocated into four groups. While oral olaparib alone This phenomenon is specific for the HR-deficient
was effective in slowing down tumor growth, application H1437 KMT2C/KD cells, as indicated by experiments
of the transdermal MNs on days 0, 5, and 10 practically with the parental cell line. As indicated in Figure 6,
blocked tumor growth in vivo, as indicated by tumor olaparib alone had a negligible effect, while combination
volume (Figure 5A) and tumor weight (Figure 5B and C). with transdermal cisplatin, though more effective, had an
These results showed that synthetic lethality is much higher effect that was less pronounced than that in the KMT2C/
when the combinatorial olaparib and cisplatin treatment KD cells, further supporting the superior efficacy of PARPi
is used. in HR-deficient cells.
Volume 9 Issue 6 (2023) 33 https://doi.org/10.36922/ijb.0048