Page 43 - IJB-9-6
P. 43
International Journal of Bioprinting Transdermal delivery of printed cisplatin
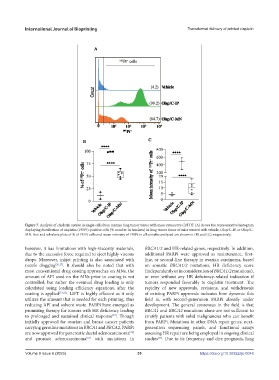
Figure 7. Analysis of cisplatin uptake in single cells from murine lung tumor tissue with mass cytometry-CyTOF. (A) shows the representative histogram
displaying distribution of cisplatin (195Pt)-positive cells (% number in brackets) in lung tumor tissue of mice treated with vehicle, Olap/C-IP, or Olap/C-
MN. Box and whiskers plots of % of 195Pt cells and mean intensity of 195Pt in all samples analyzed are shown in (B) and (C), respectively.
however, it has limitations with high-viscosity materials, BRCA1/2 and HR-related genes, respectively. In addition,
due to the excessive force required to eject highly viscous additional PARPi were approved as maintenance, first-
drops. Moreover, inkjet printing is also associated with line, or second-line therapy in ovarian carcinoma, based
nozzle clogging [26,27] . It should also be noted that with on somatic BRCA1/2 mutations, HR deficiency score
most conventional drug coating approaches on MNs, the (independently or in consideration of BRCA1/2 mutations),
amount of API used on the MNs prior to coating is not or even without any HR deficiency-related indication if
controlled, but rather the eventual drug loading is only tumors responded favorably to cisplatin treatment. The
calculated using loading efficiency equations, after the rapidity of new approvals, revisions, and withdrawals
coating is applied [51,52] . LIFT is highly efficient as it only of existing PARPi approvals indicates how dynamic this
utilizes the amount that is needed for each printing, thus field is, with second-generation PARPi already under
reducing API and solvent waste. PARPi have emerged as development. The general consensus in the field is that
promising therapy for tumors with HR deficiency leading BRCA1 and BRCA2 mutations alone are not sufficient to
to prolonged and sustained clinical response . Though stratify patients with solid malignancies who can benefit
[53]
initially approved for ovarian and breast cancer patients from PARPi. Mutations in other DNA repair genes, next-
carrying germline mutations in BRCA1 and BRCA2, PARPi generation sequencing panels, and functional assays
are now approved for pancreatic ductal adenocarcinoma assessing HR repair are being employed in ongoing clinical
[54]
and prostate adenocarcinoma with mutations in studies . Due to its frequency and dire prognosis, lung
[56]
[55]
Volume 9 Issue 6 (2023) 35 https://doi.org/10.36922/ijb.0048