Page 117 - v11i4
P. 117
International Journal of Bioprinting 3D bioprinting for translational toxicology
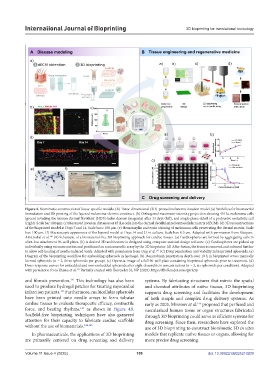
Figure 4. Biomimetic construction of tissue-specific models. (A) Three-dimensional (3D)-printed melanoma invasion model. (a) Workflow for biomaterial
formulation and 3D printing of the layered melanoma–dermis construct. (b) Orthogonal maximum-intensity projection showing 451Lu melanoma cells
(green) invading the human dermal fibroblast (HDF)-laden dermis (magenta) after 14 days (left), and single-plane detail of a protrusive metastatic cell
(right). Scale bar: 100 μm. (c) Measured invasion distances of 451Lu cells into the dermal decellularized extracellular matrix (dECM). (d) 3D reconstructions
of the bioprinted model at Days 7 and 14. Scale bars: 150 μm. (e) Hematoxylin and eosin staining of melanoma cells penetrating the dermal matrix. Scale
bar: 100 μm. (f) Macroscopic appearance of the layered model at Days 14 and 21 in culture. Scale bar: 0.5 cm. Adapted with permission from Vázquez‐
140
Aristizabal et al. (B) Schematic of a biomaterial-free 3D bioprinting approach for cardiac tissues. (a) Cardiospheres are formed by aggregating cells in
ultra-low attachment 96-well plates. (b) A desired 3D architecture is designed using computer-assisted design software. (c) Cardiospheres are picked up
individually using vacuum suction and positioned on a microneedle array by the 3D bioprinter. (d) After fusion, the tissue is removed and cultured further
143
to allow self-healing of needle-induced voids. Adapted with permission from Ong et al. (C) Drug penetration and viability in bioprinted spheroids. (a)
Diagram of the bioprinting workflow for embedding spheroids in hydrogel. (b) Doxorubicin penetration depth over 10 h in bioprinted versus manually
formed spheroids (n = 2, three spheroids per group). (c) Operetta image of a full 96-well plate containing bioprinted spheroids prior to treatment. (d)
Dose–response curves for embedded and non-embedded spheroids after eight doxorubicin concentrations (n = 2, six spheroids per condition). Adapted
147
with permission from Utama et al. Partially created with Biorender [û, NP (2025). https://BioRender.com/qvrla5y.
141
and fibrosis prevention. This technology has also been systems. By fabricating structures that mimic the spatial
used to produce hydrogel patches for treating myocardial and chemical attributes of native tissues, 3D bioprinting
infarction patients. Furthermore, multicellular spheroids supports drug screening and facilitates the development
142
have been printed onto needle arrays to form tubular of both simple and complex drug delivery systems. As
cardiac tissues to evaluate therapeutic efficacy, contractile early as 2003, Mironov et al. proposed that perfused and
146
force, and beating rhythms, as shown in Figure 4B. vascularized human tissue or organ structures fabricated
143
Scaffold-free bioprinting techniques have also garnered through 3D bioprinting could serve as efficient systems for
attention for their capacity to fabricate cardiac scaffolds drug screening. Since then, researchers have explored the
without the use of biomaterials. 144,145 use of 3D bioprinting to construct biomimetic 3D in vitro
In pharmaceuticals, the applications of 3D bioprinting models that replicate native tissues or organs, allowing for
are primarily centered on drug screening and delivery more precise drug screening.
Volume 11 Issue 4 (2025) 109 doi: 10.36922/IJB025210209