Page 19 - v11i4
P. 19
International Journal of Bioprinting 3D-printed scaffolds for osteochondral defect
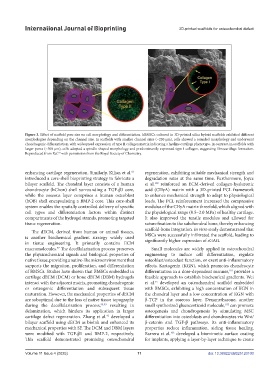
Figure 3. Effect of scaffold pore size on cell morphology and differentiation. hBMSCs cultured in 3D-printed silica hybrid scaffolds exhibited different
morphologies depending on the channel size. In scaffolds with smaller channel sizes (~230 μm), cells showed a rounded morphology and underwent
chondrogenic differentiation, with widespread expression of type II collagen matrix indicating a hyaline cartilage phenotype. In contrast, in scaffolds with
larger pores (~500 μm), cells adopted a spindle-shaped morphology and predominantly expressed type I collagen, suggesting fibrocartilage formation.
93
Reproduced from Ref. with permission from the Royal Society of Chemistry.
enhancing cartilage regeneration. Similarly, Kilian et al. regeneration, exhibiting suitable mechanical strength and
62
introduced a core-shell bioprinting strategy to fabricate a degradation rates at the same time. Furthermore, Joyce
bilayer scaffold. The chondral layer consists of a human et al. reinforced an ECM-derived collagen-hyaluronic
100
chondrocyte (hChon) shell surrounding a TGF-β3 core, acid (CHyA) matrix with a 3D-printed PCL framework
while the osseous layer comprises a human osteoblast to enhance mechanical strength to adapt to physiological
(hOB) shell encapsulating a BMP-2 core. This core-shell loads. The PCL reinforcement increased the compressive
system enables the spatially controlled delivery of specific modulus of the CHyA matrix threefold, which aligned with
cell types and differentiation factors within distinct the physiological range (0.5–2.0 MPa) of healthy cartilage.
compartments of the hydrogel strands, promoting targeted It also improved the tensile modulus and allowed for
tissue regeneration. suture fixation to the subchondral bone, thereby enhancing
scaffold-bone integration. In vitro study demonstrated that
The dECM, derived from human or animal tissues,
is another biochemical gradient strategy widely used MSCs were successfully infiltrated the scaffold, leading to
significantly higher expression of sGAG.
in tissue engineering. It primarily contains ECM
macromolecules. The decellularization process preserves Small molecules are widely applied in osteochondral
97
the physicochemical signals and biological properties of engineering to induce cell differentiation, regulate
native tissue, providing a native-like microenvironment that osteoblast/osteoclast function, or exert anti-inflammatory
supports the migration, proliferation, and differentiation effects. Kartogenin (KGN), which promotes chondrocyte
of BMSCs. Studies have shown that BMSCs embedded in differentiation in a dose-dependent manner, provides a
101
cartilage dECM (DCM) or bone dECM (DBM) hydrogels feasible approach to establish biochemical gradients. Wei
interact with the adjacent matrix, promoting chondrogenic et al. developed an osteochondral scaffold embedded
55
or osteogenic differentiation and subsequent tissue with BMSCs, exhibiting a high concentration of KGN in
maturation. However, the mechanical properties of dECM the chondral layer and a low concentration of KGN with
are suboptimal due to the loss of native tissue topography β-TCP in the osseous layer. Dexamethasone, another
during the decellularization process, 98,99 resulting in small synthesized glucocorticoid molecule, can promote
102
delamination, which hinders its application in larger osteogenesis and chondrogenesis by stimulating MSC
cartilage defect regeneration. Zhang et al. developed a differentiation into osteoblasts and chondrocytes via Wnt/
42
bilayer scaffold using dECM as bioink and enhanced its β-catenin and TGF-β pathways. Its anti-inflammatory
mechanical properties with SF. The DCM and DBM layers properties reduce inflammation, aiding tissue healing.
were modified with TGF-β1 and BMP-2, respectively. Barrera et al. developed a biomimetic surface coating
103
This scaffold demonstrated promising osteochondral for implants, applying a layer-by-layer technique to create
Volume 11 Issue 4 (2025) 11 doi: 10.36922/IJB025120100