Page 10 - IMO-2-3
P. 10
Innovative Medicines & Omics MSC exosomes for digestive tumors: Bench to bedside
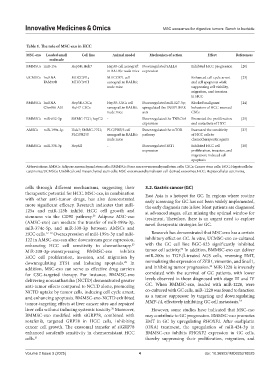
Table 1. The role of MSC‑exo in HCC
MSC‑exo Loaded small Cell line Animal model Mechanism of action Effect References
molecule
BMMSCs miR-15a Hep3B; Huh7 Hep3B cell xenograft Downregulated SALL4 Inhibited HCC progression [20]
in BALB/c nude mice expression
UCMSCs lncRNA MHCC97L; MHCC97L cell - Enhanced cell cycle arrest [23]
FAM99B MHCC97H xenograft in BALB/c and cell apoptosis while
nude mice suppressing cell viability,
migration, and invasion
in HCC
BMMSCs lncRNA Hep3B-CSCs; Hep3B-CSCs cell Downregulated miR-127-3p; Blocked malignant [24]
C5orf66-AS1 HuH7-CSCs xenograft in BALB/c upregulated the DUSP1/ERK behaviors of HCC-sourced
nude mice axis CSCs
BMMSCs miR-652-3p SMMC-7721; hepG2 - Downregulated the TNRC6A Promoted the proliferation [25]
expression and metastasis of HCC
AMSCs miR-199a-3p Huh7; SMMC-7721; PLC/PRF/5 cell Downregulated the mTOR Increased the sensitivity [27]
PLC/PRF/5 xenograft in BALB/c pathway of HCC cells to
nude mice chemotherapeutic agents
BMMSCs miR-338-3p HepG2 - Downregulated EST1 Inhibited HCC cell [28]
expression proliferation, invasion, and
migration; induced cell
apoptosis
Abbreviations: AMSCs: Adipose mesenchymal stem cells; BMMSCs: Bone marrow mesenchymal stem cells; CSCs: Cancer stem cells; HCC: Hepatocellular
carcinoma; UCMSCs: Umbilical cord mesenchymal stem cells; MSC-exo: mesenchymal stem cell-derived exosomes; HCC: Hepatocellular carcinoma.
cells through different mechanisms, suggesting their 3.2. Gastric cancer (GC)
therapeutic potential for HCC. MSC-exo, in combination East Asia is a hotspot for GC. In regions where routine
with other anti-tumor drugs, has also demonstrated early screening for GC has not been widely implemented,
more significant efficacy. Research indicates that miR- the early diagnosis rate is low. Most patients are diagnosed
125a and miR-125b inhibit HCC cell growth and at advanced stages, often missing the optimal window for
26
stemness via the CD90 pathway. Adipose MSC-exo treatment. Therefore, there is an urgent need to explore
(AMSC-exo) can mediate the transfer of miR-199a-3p, novel therapeutic strategies for GC.
miR-374c-5p, and miR-338-3p between AMSCs and
HCC cells. 27-29 Overexpression of miR-199a-3p and miR- Research has demonstrated that MSC-exo has a certain
122 in AMSC-exo can alter downstream gene expression, inhibitory effect on GC. In vitro, UCMSC-exo co-cultured
enhancing HCC cell sensitivity to chemotherapy. with the GC cell line BGC-823 significantly inhibited
27
32
MiR-338-3p-overexpressing BMMSC-exo inhibits tumor cell activity. In addition, BMMSC-exo can deliver
HCC cell proliferation, invasion, and migration by miR-200a to TGF-β-treated AGS cells, reversing EMT,
downregulating ETS1 and inducing apoptosis. In normalizing the expression of ZEB1, vimentin, and Snail1,
28
33
addition, MSC-exo can serve as effective drug carriers and inhibiting tumor progression. MiR-1228 is inversely
for CSC-targeted therapy. For instance, BMMSC-exo correlated with the survival of GC patients, with lower
delivering norcantharidin (NCTD) demonstrated greater levels observed in those diagnosed with stage III and IV
anti-tumor effects compared to NCTD alone, promoting GC. When BMMSC-exo, loaded with miR-1228, were
NCTD uptake by tumor cells, inducing cell cycle arrest, co-cultured with GC cells, miR-1228 was found to function
and enhancing apoptosis. BMMSC-exo-NCTD exhibited as a tumor suppressor by targeting and downregulating
tumor-targeting effects at liver cancer sites and repaired MMP-14, effectively inhibiting GC cell metastasis. 34
liver cells without inducing systemic toxicity. Moreover, However, some studies have indicated that MSC-exo
30
BMMSC-exo modified with siGRP78, combined with may contribute to GC progression. BMMSC-exo promotes
sorafenib, targeted GRP78 in HCC cells, inhibiting EMT in GC by upregulating RHOXF2. After oxaliplatin
cancer cell growth. The exosomal transfer of siGRP78 (OXA) treatment, the upregulation of miR-424-3p in
enhanced sorafenib sensitivity in chemoresistant HCC BMMSC-exo inhibits RHOXF2 expression in GC cells,
cells. 31 thereby suppressing their proliferation, migration, and
Volume 2 Issue 3 (2025) 4 doi: 10.36922/IMO025210025