Page 35 - IMO-2-3
P. 35
Innovative Medicines & Omics Tyrosine kinases: Structure, mechanism, and therapeutics
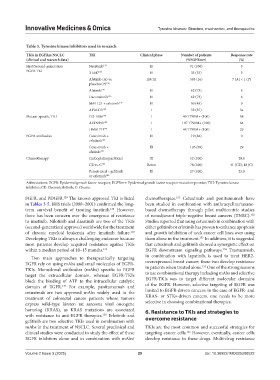
Table 3. Tyrosine kinase inhibitors used in research
TKIs in EGFRm NSCLC TKI Clinical phase Number of patients Response rate
(clinical and research data) (%EGFRm+) (%)
First/Second-generation Neratinib 132 II 91 (100) 3
EGFR TKI XL647 133 II 33 (53) 3
Afatinib (A) vs. IIB/III 585 (16) 7 (A) < 1 (P)
placebo (P) 134
Afatinib 135 II 62 (73) 8
Dacomitinib 136 II 62 (73) 8
MM-121 + erlotinib 137 II 50 (48) 9
AP26113 138 I 32 (35) 3a
Mutant-specific TKI CO-1686 139 I 40 T790M+ (100) 58
AZD9291 140 I 107 T790M+ (100) 64
HM61713 141 I 48 T790M+ (100) 29
EGFR antibodies Cetuximab + II 19 (84) 0
erlotinib 142
Cetuximab + IB 126 (98) 29
afatinib 143
Chemotherapy Carboplatin/paclitaxel III 52 (100) 28.8
CE vs. C 144 Retro 78 (100) 41 (CE); 18 (C)
Pemetrexed + gefitinib II 27 (100) 25.9
or erlotinib 145
Abbreviations: EGFR: Epidermal growth factor receptor; EGFRm+: Epidermal growth factor receptor mutation-positive; TKI: Tyrosine kinase
inhibitor; CE: Chemo/erlotinib; C: Chemo.
FGFR, and PDGFR. The known approved TKI is listed chemotherapies. Cetuximab and panitumumab have
129
151
in Tables 3-5. IRIS trials (2000–2001) confirmed the long- been studied in combination with anthracycline/taxane-
term survival benefit of treating imatinib. However, based chemotherapy through pilot multicentric studies
130
there has been concern over the emergence of resistance of neoadjuvant triple-negative breast cancers (TNBC).
152
to imatinib. Nilotinib and dasatinib are two of the TKIs Studies reported that using cetuximab in combination with
(second-generation) approved worldwide for the treatment either gefitinib or erlotinib has proven to enhance apoptosis
of chronic myeloid leukemia after imatinib failure. and growth inhibition of neck cancer cell lines over using
130
Developing TKIs is always a challenging endeavor because them alone in the treatment. In addition, it is suggested
153
most patients develop acquired resistance against TKIs that cetuximab and gefitinib showed a synergistic effect on
within a median period of 10–15 months. 131 EGFR downstream signaling pathways. Trastuzumab,
154
Two main approaches to therapeutically targeting in combination with lapatinib, is used to treat HER2-
EGFR rely on using mAbs and small molecules of EGFR- overexpressed breast cancer; these two develop resistance
155
TKIs. Monoclonal antibodies (mAbs) specific to EGFR in patients when treated alone. One of the strong reasons
target the extracellular domain, whereas EGFR-TKIs to use combinational therapy including mAbs and selective
block the binding of ATP to the intracellular catalytic EGFR-TKIs was to target different molecular domains
domain of EGFR. For example, panitumumab and of the EGFR. However, selective targeting of EGFR was
149
cetuximab are two approved mAbs widely used in the limited to EGFR-driven cancers; in the case of EGFR- and
treatment of colorectal cancer patients whose tumors KRAS- or STKs-driven cancers, one needs to be more
express wild-type kirsten rat sarcoma viral oncogene selective in choosing combinational therapies.
homolog (KRAS), as KRAS mutations are associated 6. Resistance to TKIs and strategies to
with resistance to anti-EGFR therapies. Erlotinib and overcome resistance
150
gefitinib are two selective TKIs used in combination with
mAbs in the treatment of NSCLC. Several preclinical and TKIs are the most common and successful strategies for
clinical studies were conducted to study the effect of these targeting cancer cells. However, eventually, cancer cells
156
EGFR inhibitors alone and in combination with mAbs/ develop resistance to these drugs. Multi-drug resistance
Volume 2 Issue 3 (2025) 29 doi: 10.36922/IMO025200022