Page 65 - ITPS-7-1
P. 65
INNOSC Theranostics and
Pharmacological Sciences Docking study of quinoline-3-carbaldehyde derives
burden of malaria in the year 2020 ranged between 200 functionality of HAP and thereby inhibiting the protease
and 300 million cases, resulting in approximately 627,000 of the Plasmodium parasite . By disabling HAP’s ability
[7]
deaths . Among the various species of Plasmodium to degrade hemoglobin, the propagation of the parasite
[1]
that cause malaria, Plasmodium falciparum is the most within host cells could be reduced while preserving the
formidable and widespread in Africa . In the recent hemoglobin of infected erythrocytes.
[2]
decades, drug-resistant strains of P. falciparum have Quinoline is a major component of these drugs. As an
emerged, leading to a worrisome situation where the important organic compound found in certain natural
effectiveness of currently available drugs has diminished. compounds such as alkaloids and pharmacologically
These persistent challenges have prompted the search active substances, quinoline has been reported to exhibit
for new drugs or the redesigning of existing chemical inhibitory effects on Plasmodium proteases both in vitro
compounds, aiming to establish a sustainable global public and in vivo .
[8]
health strategy.
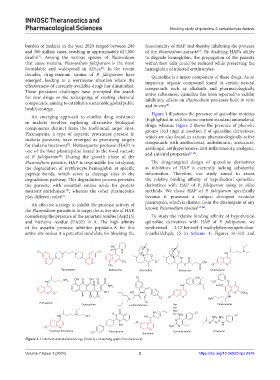
Figure 1 illustrates the presence of quinoline moieties
An emerging approach to combat drug resistance (highlighted in red) in some current standard antimalarial
in malaria involves exploring alternative biological drugs, whereas Figure 2 shows the presence of phenolic
components distinct from the traditional target sites. groups (red ring) at position 2 of quinoline derivatives,
Plasmepsins, a type of aspartic proteinase present in which are also found in various pharmacologically active
malaria parasites, have emerged as promising targets compounds with antibacterial, anthelmintic, anticancer,
for malaria treatment . Histoaspartic protease (HAP) is antifungal, antihypertensive, anti-inflammatory, analgesic,
[3]
one of the four plasmepsins found in the food vacuole and antiviral properties [9-12] .
of P. falciparum . During the growth phase of the
[4]
Plasmodium parasite, HAP is responsible for catalyzing The drug-targeted design of quinoline derivatives
the degradation of erythrocyte hemoglobin at specific as inhibitors of HAP is currently lacking substantial
peptide bonds, which serve as cleavage sites in the information. Therefore, our study aimed to assess
degradation pathway. This degradation process provides the relative binding affinity of hypothetical quinoline
the parasite with essential amino acids for protein derivatives with HAP of P. falciparum using in silico
nutrient enrichment , whereas the other plasmepsins methods. We chose HAP of P. falciparum specifically
[5]
play different roles . because it possesses a unique, divergent vacuolar
[6]
plasmepsin, which is distinct from the plasmepsin of any
An effective strategy to inhibit the protease activity of [13,14]
the Plasmodium parasite is to target the active site of HAP, known Plasmodium species .
considering the presence of the aspartate residue (Asp215) To study the relative binding affinity of hypothetical
and histidine residue (His32) in it. The high affinity quinoline derivatives with HAP of P. falciparum, we
of the aspartic protease inhibitor pepstatin-A for this synthesized 2-(2-benzoyl-4-methylphenoxy)quinoline-
active site makes it a potential candidate for blocking the 3-carbaldehyde (5 in Scheme 1; Figures S1–S3) and
Figure 1. Common antimalarial drugs (mostly containing quinoline moieties).
Volume 7 Issue 1 (2024) 2 https://doi.org/10.36922/itps.0976