Page 67 - ITPS-8-2
P. 67
INNOSC Theranostics and
Pharmacological Sciences Preclinical study of GBpoietin biosimilar
A B
C D
E F
G H
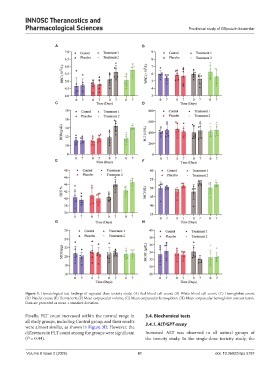
Figure 3. Hematological test findings of repeated-dose toxicity study. (A) Red blood cell count; (B) White blood cell count; (C) Hemoglobin count;
(D) Platelet count; (E) Hematocrit; (F) Mean corpuscular volume; (G) Mean corpuscular hemoglobin; (H) Mean corpuscular hemoglobin concentration.
Data are presented as mean ± standard deviation.
Finally, PLT count increased within the normal range in 3.4. Biochemical tests
all study groups, including Control group, and their results 3.4.1. ALT/GPT assay
were almost similar, as shown in Figure 3D. However, the
differences in PLT count among the groups were significant Increased ALT was observed in all animal groups of
(P = 0.44). the toxicity study. In the single-dose toxicity study, the
Volume 8 Issue 2 (2025) 61 doi: 10.36922/itps.5797