Page 47 - JCTR-10-5
P. 47
Arnold and Arm | Journal of Clinical and Translational Research 2024;10(5):307-316 309
use and can be topically applied in a dry form to the surface of models of pressure injury and ischemia [12,13]. In a robust
the wound (Figure 1). PMVT is isolated through a proprietary Level 1, prospective, randomized, controlled, and multicenter
process that involves cutting, cleaning, isolation, lyophilization, clinical trial involving 100 diabetic patients with non-healing
and sterilization of the harvested tissue, and is intended to Wagner Grades 1 and 2 neuropathic foot ulcers (the “HIFLO
improve blood flow through the repair and reconstruction Trial”), the weekly topical application of PMVT resulted in
of microvascular tissue, by serving as a scaffold for cellular significantly increased complete wound closure at 12 weeks
invasion and capillary growth. The benefits of improved compared to the standard-of-care group (74% vs. 38%; P =
microcirculatory blood flow may be particularly impactful on 0.00029, with a nine-fold increased odds of healing). Sub-
patients with compromised microvasculature. studies also demonstrated improved wound area perfusion
Preclinical studies of PMVT demonstrated angiogenesis and increased levels of sensation and tissue quality in this
support and significantly increased healing rates in rodent neuropathic patient population [14,15]. Here, we report on
real-world clinical experiences with PMVT in a case series of
A five challenging non-healing wounds.
2. Methods
All patients received weekly or semi-weekly topical PMVT
treatment until wound sites closed or demonstrated active
healing with evidence of good microcirculation and progressing
re-epithelialization. In all cases within this series, PMVT
was covered with a non-adherent dressing and left untouched
between visits. Patients were directed not to change the wound
dressing, to comply with standard care guidance appropriate for
each of their wounds, and to return weekly for assessment of
the wound and (if needed) reapplication of the PMVT product.
Wound size was measured using a ruler at each visit, and, when
appropriate, wound depth was determined using a DM Stick
foam-tipped measuring device (Puritan, USA). Closure criteria
were 100% epithelialization with no maceration, exudate, or
signs of infection.
As this case series was conducted under the standard
practice of medicine with a commercial human tissue
product for each respective application, no additional ethical
regulations or formal research protocol were required, nor were
the cases added to a public database. All facility procedures
for obtaining patient consent for treatment were followed,
and release forms to allow data and image publication were
obtained from each patient.
3. Results
B
3.1. Case 1: Stimulation of perfusion and healing using PMVT
in a refractory metatarsal diabetic foot ulcer
Despite growing efforts to adopt a “limb preservation”
approach in wound clinics, amputations are an increasingly
unwanted complication of non-healing foot ulcers in diabetic
patients and are known to have a 50% mortality rate within
5 years [16]. When amputation is necessary, a transmetatarsal
amputation (TMA) (as opposed to a below-the-knee amputation)
may be justified when macrovascular blood flow to the foot is
sufficient. However, patients with TMA are at high risk of skin
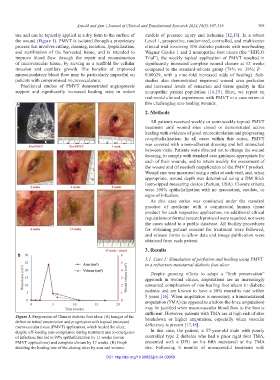
Figure 3. Progression of Charcot diabetic foot ulcer. (A) Images of the
defect on initial presentation and progression with topical processed breakdown or higher amputation, especially when vascular
microvascular tissue (PMVT) application, which healed the ulcer, deficiency is present [17,18].
despite off-loading non-compliance during treatment and re-emergence In this case, the patient, a 57-year-old male with poorly
of infection; this led to 99% epithelialization by 11 weeks (seven controlled type 2 diabetes who had a prior right foot TMA,
PMVT applications) and complete closure by 17 weeks. (B) Graph presented with a DFU on his fifth metatarsal at the TMA
detailing the healing rate of the closing ulcer by area and volume. site. Following 6 months of unsuccessful treatment with
DOI: http://doi.org/10.36922/jctr.24.00059