Page 31 - TD-4-2
P. 31
Tumor Discovery Understanding glioblastoma invasion and therapy
of a stereotactic tumor biopsy, is required to diagnose Table 1. World Health Organization central nervous system
GBM definitively. Tissue is most commonly collected tumor grading system
during standard-of-care tumor resection operations. Grade Description
The assignment of a GBM diagnosis was historically
based on histopathological and gross tissue evaluation. Grade 1 • Slow‑growing, benign
Grossly, GBM is a poorly demarcated, vascularized, and Grade 2 • Relatively slow growing
friable tumor that commonly exhibits a grayish rim of • May be malignant or benign
• Commonly recur as higher‑grade tumors
rubbery tissue with a central area of yellow-tan necrosis
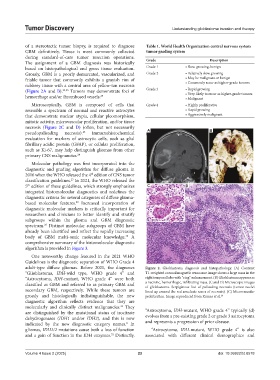
(Figure 2A and B). 24,25 Tumors may demonstrate foci of Grade 3 • Rapid growing
• Very likely to recur as higher‑grade tumors
hemorrhage and/or thrombosed vessels. 25 • Malignant
Microscopically, GBM is composed of cells that Grade 4 • Highly proliferative
resemble a spectrum of normal and reactive astrocytes • Rapid growing
that demonstrate nuclear atypia, cellular pleomorphism, • Aggressively malignant
mitotic activity, microvascular proliferation, and/or tissue
necrosis (Figure 2C and D) (often, but not necessarily A
pseudopalisading necrosis). 25 Immunohistochemical B
evaluation for markers of astrocytic cells, such as glial
fibrillary acidic protein (GFAP), or cellular proliferation,
such as Ki-67, may help distinguish gliomas from other
primary CNS malignancies. 24
Molecular pathology was first incorporated into the
diagnostic and grading algorithm for diffuse glioma in
2016 when the WHO released the 4 edition of CNS tumor
th
classification guidelines. In 2021, the WHO released the
27
5 edition of these guidelines, which strongly emphasizes C D
th
integrated histomolecular diagnostics and redefines the
diagnostic criteria for several categories of diffuse glioma-
based molecular features. Increased incorporation of
23
diagnostic molecular markers is critically important for
researchers and clinicians to better identify and stratify
subgroups within the glioma and GBM diagnostic
spectrum. Distinct molecular subgroups of GBM have
28
already been identified and reflect the rapidly increasing
body of GBM multi-omic molecular knowledge. A
29
comprehensive summary of the histomolecular diagnostic
algorithm is provided in Figure 3.
One noteworthy change featured in the 2021 WHO
Guidelines is the diagnostic separation of WHO Grade 4
adult-type diffuse gliomas. Before 2021, the diagnoses Figure 2. Glioblastoma diagnosis and histopathology. (A) Contrast
“Glioblastoma, IDH-wild type, WHO grade 4” and T1-weighted coronal magnetic resonance image shows a large mass in the
“Astrocytoma, IDH-mutant, WHO grade 4” were both right temporal lobe with “ring” enhancement. (B) Glioblastoma appears as
classified as GBM and referred to as primary GBM and a necrotic, hemorrhagic, infiltrating mass. (C and D) Microscopic images
of glioblastoma. Serpiginous foci of palisading necrosis (tumor nuclei
secondary GBM, respectively. While these tumors are lined up around the red anucleate zones of necrosis). (C) Microvascular
grossly and histologically indistinguishable, the new proliferation. Image reproduced from Kumar et al. 26
diagnostic algorithm reflects evidence that they are
molecularly and clinically distinct malignancies. They
31
are distinguished by the mutational status of isocitrate “Astrocytoma, IDH-mutant, WHO grade 4” typically (d)
dehydrogenases (IDH1 and/or IDH2), and this is now evolves from a pre-existing grade 2 or grade 3 astrocytoma
indicated by the new diagnostic category names. In and represents a progression of prior disease.
8
gliomas, IDH1/2 mutations cause both a loss of function “Astrocytoma, IDH-mutant, WHO grade 4” is also
and a gain of function in the IDH enzymes. Distinctly, associated with different clinical demographics and
32
Volume 4 Issue 2 (2025) 23 doi: 10.36922/td.8578