Page 82 - TD-4-3
P. 82
Tumor Discovery Mg-28-A theoretical novel strategy in cancer therapy
A B
C D
E F
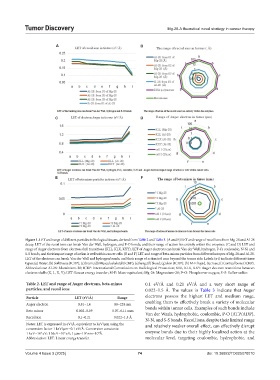
Figure 1. LET and range of different particles in biological tissues, derived from Table 2 and Table 3. (A and B) LET and range of recoil ions from Mg-28 and Al-28
decay. LET of the recoil ions can break Van der Wall, hydrogen, and P-O bonds, and their range of action lies entirely within the enzymes. (C and D) LET and
range of Auger electrons from electron shell transitions (KLL, KLX, KXY). LET of Auger electrons can break Van der Wall, hydrogen, P-O, coulombic, N-N, and
S-S bonds, and their impact range of action is well within cancer cells. (E and F) LET and range of beta-minus particles from different isotopes of Mg-28 and Al-28.
LET of the electrons can break Van der Wall and hydrogen bonds, and their range of action is 6 mm beyond the tumor side. Labels (a-i) indicate different tissue
types: (a) Water; (b) Soft tissue (ICRP); (c) brain; (d) Musculoskeletal (ICRP); (e) lung; (f) blood; (g) skin (ICRP); (h) M-E liquid, Sucrose; (i) Cortical bone (ICRP).
Abbreviations: Al-28: Aluminium-28; ICRP: International Commission on Radiological Protection; KLL, KLX, KXY: Auger electron transitions between
electron shells (K, L, X, Y); LET: Linear energy transfer; M-E: Mass-equivalent; Mg-28: Magnesium-28; P–O: Phosphorus-oxygen; S–S: Sulfur-sulfur.
Table 2. LET and range of Auger electrons, beta‑minus 0.1 eV/Å and 0.21 eV/Å and a very short range of
particles, and recoil ions 0.022–1.5 Å. The values in Table 3 indicate that Auger
Particle LET (eV/Å) Range electrons possess the highest LET and medium range,
Auger electron 0.81–1.6 88–224 nm enabling them to effectively break a variety of molecular
Beta minus 0.002–0.09 0.07–6.11 mm bonds within tumor cells. Examples of such bonds include
Van der Waals, hydrophobic, coulombic, P-O (ATP/ADP),
Recoil ion 0.1–0.21 0.022–1.5 Å N-N, and S-S bonds. Recoil ions, despite their limited range
Notes: LET is expressed in eV/Å, equivalent to keV/μm using the and relatively weaker overall effect, can effectively disrupt
conversion factor 1 keV/μm=0.1 eV/Å. Conversion constants:
1 keV=10 eV; 1 MeV=10 eV; 1 μm=10 nm=10 Å. enzyme bonds due to their highly localized action at the
3
4
6
3
Abbreviation: LET: Linear energy transfer. molecular level, targeting coulombic, hydrophobic, and
Volume 4 Issue 3 (2025) 74 doi: 10.36922/TD025070010