Page 208 - AJWEP-22-4
P. 208
Shrestha
commercial AC were evaluated under identical batch
conditions (20 ppm RhB, pH 8.5, 0.03 g adsorbent,
room temperature). The normalized concentration ratio
(C/C₀) was plotted as a function of time to assess the
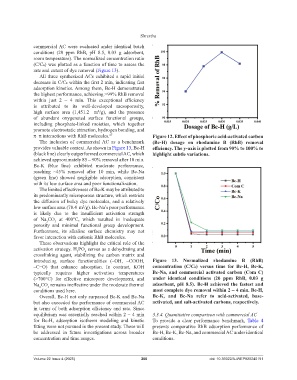
rate and extent of dye removal (Figure 13).
All three synthesized ACs exhibited a rapid initial
decrease in C/C₀ within the first 2 min, indicating fast
adsorption kinetics. Among them, Bc-H demonstrated
the highest performance, achieving >99% RhB removal
within just 2 – 4 min. This exceptional efficiency
is attributed to its well-developed mesoporosity,
high surface area (1,451.2 m /g), and the presence
2
of abundant oxygenated surface functional groups,
including phosphate-linked moieties, which together
promote electrostatic attraction, hydrogen bonding, and
π–π interactions with RhB molecules. 27 Figure 12. Effect of phosphoric acid-activated carbon
The inclusion of commercial AC as a benchmark (Bc-H) dosage on rhodamine B (RhB) removal
provides valuable context. As shown in Figure 13, Bc-H efficiency. The y-axis is plotted from 90% to 100% to
(black line) clearly outperformed commercial AC, which highlight subtle variations.
achieved approximately 85 – 90% removal after 10 min.
Bc-K (blue line) exhibited moderate performance,
reaching ~45% removal after 10 min, while Bc-Na
(green line) showed negligible adsorption, consistent
with its low surface area and poor functionalization.
The limited effectiveness of Bc-K may be attributed to
its predominantly microporous structure, which restricts
the diffusion of bulky dye molecules, and a relatively
low surface area (78.4 m /g). Bc-Na’s poor performance
2
is likely due to the insufficient activation strength
of Na CO at 400°C, which resulted in inadequate
2
3
porosity and minimal functional group development.
Furthermore, its alkaline surface chemistry may not
favor interaction with cationic RhB molecules.
These observations highlight the critical role of the
activation strategy. H PO serves as a dehydrating and
3
4
crosslinking agent, stabilizing the carbon matrix and
introducing surface functionalities (–OH, –COOH, Figure 13. Normalized rhodamine B (RhB)
–C=O) that enhance adsorption. In contrast, KOH concentration (C/C₀) versus time for Bc-H, Bc-K,
typically requires higher activation temperatures Bc-Na, and commercial activated carbon (Com C)
(>700°C) for effective micropore development, and under identical conditions (20 ppm RhB, 0.03 g
Na CO remains ineffective under the moderate thermal adsorbent, pH 8.5). Bc-H achieved the fastest and
3
2
conditions used here. most complete dye removal within 2 – 4 min. Bc-H,
Overall, Bc-H not only surpassed Bc-K and Bc-Na Bc-K, and Bc-Na refer to acid-activated, base-
but also exceeded the performance of commercial AC activated, and salt-activated carbons, respectively.
in terms of both adsorption efficiency and rate. Since
equilibrium was essentially reached within 2 – 4 min 3.3.4. Quantitative comparison with commercial AC
for Bc-H, adsorption isotherm modeling and kinetic To provide a clear performance benchmark, Table 4
fitting were not pursued in the present study. These will presents comparative RhB adsorption performance of
be addressed in future investigations across broader Bc-H, Bc-K, Bc-Na, and commercial AC under identical
concentration and time ranges. conditions.
Volume 22 Issue 4 (2025) 200 doi: 10.36922/AJWEP025240191