Page 206 - AJWEP-22-4
P. 206
Shrestha
adsorption orientation, mobility, and affinity toward
charged surfaces. In addition, changes in polarity
may influence the extent of self-aggregation in
solution.
At very high pH (above 10), aggregation, micelle-
like behavior, or partial precipitation may occur
due to reduced solubility, which lowers the
concentration of freely available dye molecules for
adsorption. Excess hydroxide ions (OH⁻) may also
16
compete with RhB for active adsorption sites on the
adsorbent surface.
In terms of surface charges, the surface charge of
Bc-H is primarily governed by the protonation
and deprotonation of its surface functional groups,
particularly –OH, –COOH, and –C=O, introduced
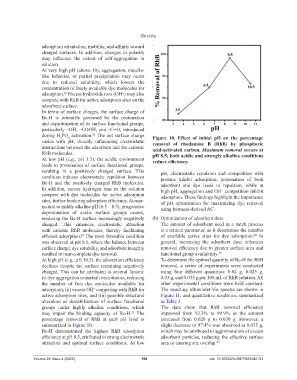
during H PO activation. The net surface charge Figure 10. Effect of initial pH on the percentage
23
4
3
varies with pH, directly influencing electrostatic removal of rhodamine B (RhB) by phosphoric
interactions between the adsorbent and the cationic acid-activated carbon. Maximum removal occurs at
RhB molecules. pH 8.5; both acidic and strongly alkaline conditions
At low pH (e.g., pH 3.5), the acidic environment reduce efficiency.
leads to protonation of surface functional groups,
resulting in a positively charged surface. This pH, electrostatic repulsion and competition with
condition induces electrostatic repulsion between protons inhibit adsorption; protonation of both
Bc-H and the positively charged RhB molecules. adsorbent and dye leads to repulsion, while at
In addition, excess hydrogen ions in the solution high pH, aggregation and OH⁻ competition inhibit
compete with dye molecules for active adsorption adsorption. These findings highlight the importance
sites, further hindering adsorption efficiency. At near- of pH optimization for maximizing dye removal
neutral to mildly alkaline pH (6.5 – 8.5), progressive using biomass-derived AC.
deprotonation of acidic surface groups occurs,
rendering the Bc-H surface increasingly negatively (b) Optimization of adsorbent dose
charged. This enhances electrostatic attraction The amount of adsorbent used in a batch process
with cationic RhB molecules, thereby facilitating is a critical parameter, as it determines the number
efficient adsorption. The most favorable condition of available active sites for dye adsorption. In
24
24
was observed at pH 8.5, where the balance between general, increasing the adsorbent dose enhances
surface charge, dye solubility, and adsorbent integrity removal efficiency due to greater surface area and
resulted in near-complete dye removal. functional group availability. 19
At high pH (e.g., pH 10.5), the adsorption efficiency To determine the optimal quantity of Bc-H for RhB
declines despite the surface remaining negatively removal, a series of experiments were conducted
charged. This can be attributed to several factors: using four different quantities: 0.02 g, 0.025 g,
(i) dye aggregation or partial precipitation, reducing 0.03 g, and 0.035 g per 100 mL of RhB solution. All
the number of free dye molecules available for other experimental conditions were held constant.
adsorption, (ii) excess OH⁻ competing with RhB for The resulting ultraviolet-Vis spectra are shown in
active adsorption sites, and (iii) possible structural Figure 11, and quantitative results are summarized
alteration or destabilization of surface functional in Table 3.
groups under highly alkaline conditions, which The data show that RhB removal efficiency
may impair the binding capacity of Bc-H. The improved from 92.3% to 99.9% as the amount
25
percentage removal of RhB at each pH level is increased from 0.020 g to 0.030 g. However, a
summarized in Figure 10. slight decrease to 97.4% was observed at 0.035 g,
Bc-H demonstrated the highest RhB adsorption which may be attributed to agglomeration of excess
efficiency at pH 8.5, attributed to strong electrostatic adsorbent particles, reducing the effective surface
attraction and optimal surface conditions. At low area or causing site overlap. 26
Volume 22 Issue 4 (2025) 198 doi: 10.36922/AJWEP025240191