Page 202 - AJWEP-22-4
P. 202
Shrestha
most developed and uniform mesoporous structure,
attributed to the efficient activating effect of H PO 4
3
at 400°C. In contrast, Bc-K and Bc-Na exhibited
suboptimal activation, with denser or irregular surfaces
due to insufficient thermal conditions for their respective
activating chemistries. These morphological trends
are consistent with BET surface areas and structural
disorder observed through Raman and XRD, reinforcing
the superior activation achieved in Bc-H.
While SEM revealed the general surface morphology,
higher-resolution imaging (e.g., transmission electron
microscopy) could further elucidate nanostructural
features and will be considered in future studies.
3.2.4. Nitrogen adsorption/desorption isotherm and
BET surface area analysis
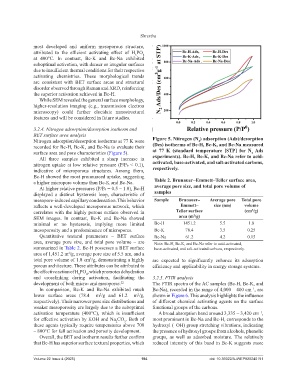
Nitrogen adsorption/desorption isotherms at 77 K were Figure 5. Nitrogen (N ) adsorption (Ads)/desorption
2
recorded for Bc-H, Bc-K, and Bc-Na to evaluate their (Des) isotherms of Bc-H, Bc-K, and Bc-Na measured
surface area and pore characteristics (Figure 5). at 77 K (standard temperature [STP] for N Ads
2
All three samples exhibited a sharp increase in experiments). Bc-H, Bc-K, and Bc-Na refer to acid-
nitrogen uptake at low relative pressure (P/P₀ < 0.1), activated, base-activated, and salt-activated carbons,
indicative of microporous structures. Among them, respectively.
Bc-H showed the most pronounced uptake, suggesting
a higher micropore volume than Bc-K and Bc-Na. Table 2. Brunauer–Emmett–Teller surface area,
At higher relative pressures (P/P₀ = 0.5 – 1.0), Bc-H average pore size, and total pore volume of
displayed a distinct hysteresis loop, characteristic of samples
mesopore-induced capillary condensation. This behavior Sample Brunauer– Average pore Total pore
reflects a well-developed mesoporous network, which Emmett– size (nm) volume
3
correlates with the highly porous surface observed in Teller surface (cm /g)
2
SEM images. In contrast, Bc-K and Bc-Na showed area (m /g)
minimal or no hysteresis, implying more limited Bc-H 1451.2 5.5 1.8
mesoporosity and a predominance of micropores. Bc-K 78.4 3.5 0.25
Quantitative textural parameters – BET surface Bc-Na 61.2 4.2 0.15
area, average pore size, and total pore volume – are Note: Bc-H, Bc-K, and Bc-Na refer to acid-activated,
summarized in Table 2. Bc-H possesses a BET surface base-activated, and salt-activated carbons, respectively.
area of 1,451.2 m /g, average pore size of 5.5 nm, and a
2
total pore volume of 1.8 cm /g, demonstrating a highly are expected to significantly enhance its adsorption
3
porous architecture. These attributes can be attributed to efficiency and applicability in energy storage systems.
the effective action of H PO , which promotes dehydration
3
4
and crosslinking during activation, facilitating the 3.2.5. FTIR analysis
development of both micro- and mesopores. 22 The FTIR spectra of the AC samples (Bc-H, Bc-K, and
In comparison, Bc-K and Bc-Na exhibited much Bc-Na), recorded in the range of 4,000 – 400 cm , are
−1
lower surface areas (78.4 m /g and 61.2 m /g, shown in Figure 6. This analysis highlights the influence
2
2
respectively). Their narrower pore size distributions and of different chemical activating agents on the surface
weaker mesoporosity are largely due to the suboptimal functional groups of the carbons.
activation temperature (400°C), which is insufficient A broad absorption band around 3,335 – 3,420 cm ,
−1
for effective activation by KOH and Na CO . Both of most prominent in Bc-Na and Bc-H, corresponds to the
2
3
these agents typically require temperatures above 700 hydroxyl (–OH) group stretching vibrations, indicating
– 800°C for full activation and porosity development. the presence of hydroxyl groups from alcohols, phenolic
Overall, the BET and isotherm results further confirm groups, as well as adsorbed moisture. The relatively
that Bc-H has superior surface textural properties, which reduced intensity of this band in Bc-K suggests more
Volume 22 Issue 4 (2025) 194 doi: 10.36922/AJWEP025240191