Page 200 - AJWEP-22-4
P. 200
Shrestha
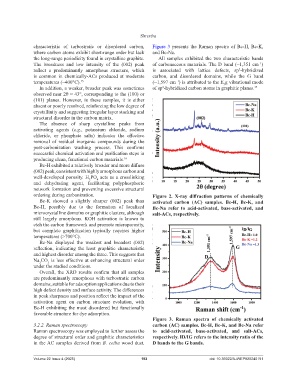
characteristic of turbostratic or disordered carbon, Figure 3 presents the Raman spectra of Bc-H, Bc-K,
where carbon atoms exhibit short-range order but lack and Bc-Na.
the long-range periodicity found in crystalline graphite. All samples exhibited the two characteristic bands
The broadness and low intensity of the (002) peak of carbonaceous materials. The D band (~1,351 cm )
−1
reflect a predominantly amorphous structure, which is associated with lattice defects, sp -hybridized
3
is common in chemically-ACs produced at moderate carbon, and disordered domains, while the G band
temperatures (~400°C). 15 (~1,597 cm ) is attributed to the E g vibrational mode
−1
2
In addition, a weaker, broader peak was sometimes of sp -hybridized carbon atoms in graphitic planes. 17
2
observed near 2θ ≈ 43°, corresponding to the (100) or
(101) planes. However, in these samples, it is either
absent or poorly resolved, reinforcing the low degree of
crystallinity and suggesting irregular layer stacking and
structural disorder in the carbon matrix.
The absence of sharp crystalline peaks from
activating agents (e.g., potassium chloride, sodium
chloride, or phosphate salts) indicates the effective
removal of residual inorganic compounds during the
post-carbonization washing process. This confirms
successful chemical activation and purification steps in
producing clean, functional carbon materials. 16
Bc-H exhibited a relatively broader and more diffuse
(002) peak, consistent with highly amorphous carbon and
well-developed porosity. H PO acts as a crosslinking
4
3
and dehydrating agent, facilitating polyphosphoric
network formation and preventing excessive structural
ordering during carbonization. Figure 2. X-ray diffraction patterns of chemically
Bc-K showed a slightly sharper (002) peak than activated carbon (AC) samples. Bc-H, Bc-K, and
Bc-H, possibly due to the formation of localized Bc-Na refer to acid-activated, base-activated, and
microcrystalline domains or graphitic clusters, although salt-ACs, respectively.
still largely amorphous. KOH activation is known to
etch the carbon framework and promote microporosity,
but complete graphitization typically requires higher
temperatures (>700°C).
Bc-Na displayed the weakest and broadest (002)
reflection, indicating the least graphitic characteristic
and highest disorder among the three. This suggests that
Na CO is less effective at enhancing structural order
2
3
under the studied conditions.
Overall, the XRD results confirm that all samples
are predominantly amorphous with turbostratic carbon
domains, suitable for adsorption applications due to their
high defect density and surface activity. The differences
in peak sharpness and position reflect the impact of the
activation agent on carbon structure evolution, with
Bc-H exhibiting the most disordered but functionally
favorable structure for dye adsorption.
Figure 3. Raman spectra of chemically activated
3.2.2. Raman spectroscopy carbon (AC) samples. Bc-H, Bc-K, and Bc-Na refer
Raman spectroscopy was employed to further assess the to acid-activated, base-activated, and salt-ACs,
degree of structural order and graphitic characteristics respectively. ID/IG refers to the intensity ratio of the
in the AC samples derived from B. ceiba wood dust. D bands to the G bands.
Volume 22 Issue 4 (2025) 192 doi: 10.36922/AJWEP025240191