Page 204 - AJWEP-22-4
P. 204
Shrestha
3.3.1. Comparative performance of chemically-ACs exceeded the performance of commercial AC, making
and commercially-AC it a strong candidate for practical wastewater treatment
Before analyzing parameter-specific adsorption trends, applications.
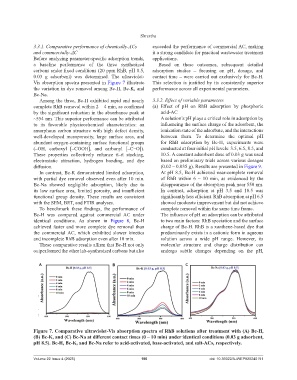
a baseline performance of the three synthesized Based on these outcomes, subsequent detailed
carbons under fixed conditions (20 ppm RhB, pH 8.5, adsorption studies – focusing on pH, dosage, and
0.03 g adsorbent) was determined. The ultraviolet- contact time – were carried out exclusively for Bc-H.
Vis absorption spectra presented in Figure 7 illustrate This selection is justified by its consistently superior
the variation in dye removal among Bc-H, Bc-K, and performance across all experimental parameters.
Bc-Na.
Among the three, Bc-H exhibited rapid and nearly 3.3.2. Effect of variable parameters
complete RhB removal within 2 – 4 min, as confirmed (a) Effect of pH on RhB adsorption by phosphoric
by the significant reduction in the absorbance peak at acid-AC
~554 nm. This superior performance can be attributed A solution’s pH plays a critical role in adsorption by
to its favorable physicochemical characteristics: an influencing the surface charge of the adsorbent, the
amorphous carbon structure with high defect density, ionization state of the adsorbate, and the interactions
well-developed mesoporosity, large surface area, and between them. To determine the optimal pH
abundant oxygen-containing surface functional groups for RhB adsorption by Bc-H, experiments were
(–OH, carboxyl [–COOH], and carbonyl [–C=O]). conducted at four initial pH levels: 3.5, 6.5, 8.5, and
These properties collectively enhance π–π stacking, 10.5. A constant adsorbent dose of 0.03 g was used
electrostatic attraction, hydrogen bonding, and dye based on preliminary trials across various dosages
diffusion. (0.02 – 0.035 g). Results are presented in Figure 9.
In contrast, Bc-K demonstrated limited adsorption, At pH 8.5, Bc-H achieved near-complete removal
with partial dye removal observed even after 10 min. of RhB within 6 – 10 min, as evidenced by the
Bc-Na showed negligible adsorption, likely due to disappearance of the absorption peak near 550 nm.
its low surface area, limited porosity, and insufficient In contrast, adsorption at pH 3.5 and 10.5 was
functional group density. These results are consistent significantly less efficient. RhB absorption at pH 6.5
with the SEM, BET, and FTIR analyses. showed moderate improvement but did not achieve
To benchmark these findings, the performance of complete removal within the same time frame.
Bc-H was compared against commercial AC under The influence of pH on adsorption can be attributed
identical conditions. As shown in Figure 8, Bc-H to two main factors: RhB speciation and the surface
achieved faster and more complete dye removal than charge of Bc-H. RhB is a xanthene-based dye that
the commercial AC, which exhibited slower kinetics predominantly exists in a cationic form in aqueous
and incomplete RhB adsorption even after 10 min. solution across a wide pH range. However, its
These comparative results affirm that Bc-H not only molecular structure and charge distribution can
outperformed the other lab-synthesized carbons but also undergo subtle changes depending on the pH,
A B C
Figure 7. Comparative ultraviolet-Vis absorption spectra of RhB solutions after treatment with (A) Bc-H,
(B) Bc-K, and (C) Bc-Na at different contact times (0 – 10 min) under identical conditions (0.03 g adsorbent,
pH 8.5). Bc-H, Bc-K, and Bc-Na refer to acid-activated, base-activated, and salt-ACs, respectively.
Volume 22 Issue 4 (2025) 196 doi: 10.36922/AJWEP025240191