Page 129 - AJWEP-22-6
P. 129
Plantain waste for lab water cleanup
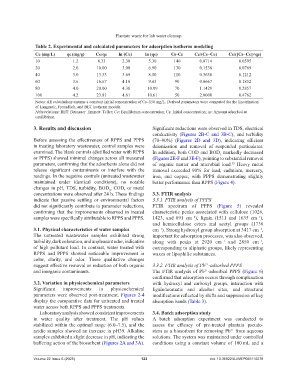
Table 2. Experimental and calculated parameters for adsorption isotherm modeling
Ce (mg/L) qe(mg/g) Ce/qe ln (Ce) ln (qe) Cs−Ce Ce/(Cs−Ce) Ce/([Cs−Ce]×qe)
10 1.2 8.33 2.30 5.30 140 0.0714 0.0595
20 2.0 10.00 3.00 6.90 130 0.1538 0.0769
40 3.0 13.33 3.69 8.50 110 0.3636 0.1212
60 3.6 16.67 4.10 9.43 90 0.6667 0.1852
80 4.0 20.00 4.38 10.09 70 1.1429 0.2857
100 4.2 23.81 4.61 10.61 50 2.0000 0.4762
Notes: All calculations assume a constant initial concentration of Cs=150 mg/L. Derived parameters were computed for the linearization
of Langmuir, Freundlich, and BET isotherm models.
Abbreviations: BET: Brunauer–Emmett–Teller; Ce: Equilibrium concentration; Cs: Initial concentration; qe: Amount adsorbed at
equilibrium.
3. Results and discussion Significant reductions were observed in TDS, electrical
conductivity (Figures 2B-C and 3B-C), and turbidity
Before assessing the effectiveness of RPPS and PPPS (70–90%) (Figures 2D and 3D), indicating efficient
in treating laboratory wastewater, control samples were deionization and removal of suspended particulates.
examined. The blank controls (distilled water with RPPS In addition, both COD and BOD markedly decreased
5
or PPPS) showed minimal changes across all measured (Figures 2E-F and 3E-F), pointing to substantial removal
parameters, confirming that the adsorbents alone did not of organic matter and microbial load. Heavy metal
35
release significant contaminants or interfere with the removal exceeded 90% for lead, cadmium, mercury,
readings. In the negative controls (untreated wastewater iron, and copper, with PPPS demonstrating slightly
maintained under identical conditions), no notable better performance than RPPS (Figure 4).
changes in pH, TDS, turbidity, BOD , COD, or metal
5
concentrations were observed after 24 h. These findings 3.3. FTIR analysis
indicate that passive settling or environmental factors 3.3.1. FTIR analysis of PPPS
did not significantly contribute to parameter reductions, FTIR spectrum of PPPS (Figure 5) revealed
confirming that the improvements observed in treated characteristic peaks associated with cellulose (1026,
samples were specifically attributable to RPPS and PPPS. 1423, and 891 cm⁻¹), lignin (1511 and 1635 cm⁻¹),
and hemicellulose esters and acetyl groups (1736
3.1. Physical characteristics of water samples cm⁻¹). Strong hydroxyl group absorption at 3417 cm⁻¹,
The untreated wastewater samples exhibited strong important for adsorption processes, was also observed,
turbidity, dark coloration, and unpleasant odor, indicative along with peaks at 2920 cm⁻¹ and 2850 cm⁻¹,
of high pollutant load. In contrast, water treated with corresponding to aliphatic groups, likely representing
RPPS and PPPS showed noticeable improvement in waxes or lipophilic substances.
color, clarity, and odor. These qualitative changes
suggest effective removal or reduction of both organic 3.3.2. FTIR analysis of Pb -adsorbed PPPS
2+
and inorganic contaminants. The FTIR analysis of Pb²⁺-adsorbed PPPS (Figure 6)
confirmed that adsorption occurs through complexation
3.2. Variation in physicochemical parameters with hydroxyl and carboxyl groups, interaction with
Significant improvements in physicochemical lignin/aromatic and alcohol sites, and structural
parameters were observed post-treatment. Figures 2-4 modifications reflected by shifts and suppression of key
display the comparative data for untreated and treated absorption bands (Table 3).
water across both RPPS and PPPS treatments.
Laboratory analysis showed consistent improvements 3.4. Batch adsorption study
in water quality after treatment. The pH values A batch adsorption experiment was conducted to
stabilized within the optimal range (6.0–7.5), and the assess the efficacy of pre-treated plantain pseudo-
acidic samples showed an increase in pH39. Alkaline stem as a biosorbent for removing Pb²⁺ from aqueous
samples exhibited a slight decrease in pH, indicating the solutions. The system was maintained under controlled
buffering action of the biosorbent (Figures 2A and 3A). conditions using a constant volume of 100 mL and a
Volume 22 Issue 6 (2025) 123 doi: 10.36922/AJWEP025110078