Page 145 - {PDF Title}
P. 145
Groundwater quality in Patna District
The Piper diagram presented provides a graphical silicate minerals. The predominant anions are HCO
−
3
representation of groundwater chemistry in the Patna and CO , signifying bicarbonate-type water commonly
−
3
district (Figure 2). Most of the groundwater samples associated with carbonate rock dissolution. Some
show a dominance of Ca and Mg , indicating the samples also exhibit Cl and SO dominance, indicating
2+
2+
2−
−
4
influence of carbonate minerals such as limestone or potential anthropogenic influences (e.g., agricultural
dolomite. Some samples are enriched in Na and K , an runoff, industrial discharge) or evaporite dissolution.
+
+
indication of ion exchange processes or interaction with Most of the samples fall in the Ca -Mg -HCO region,
2+
2+
−
3
indicating fresh groundwater influenced by carbonate
weathering. A few samples trend toward the Na -Cl
−
+
and SO zones, suggesting mixing with saline water,
2−
4
possible anthropogenic contamination, or groundwater
evolution due to ion exchange. The dominance of Ca -
2+
Mg -HCO water type is typical of groundwater in
−
2+
3
areas influenced by carbonate rocks. The presence of
Cl and SO -rich samples indicates potential salinity
2−
−
4
issues or pollution sources.
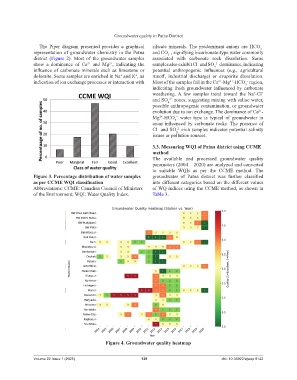
3.3. Measuring WQI of Patna district using CCME
method
The available and processed groundwater quality
parameters (2004 – 2020) are analyzed and converted
to suitable WQIs as per the CCME method. The
Figure 3. Percentage distribution of water samples groundwater of Patna district was further classified
as per CCME WQI classification into different categories based on the different values
Abbreviations: CCME: Canadian Council of Ministers of WQ indices using the CCME method, as shown in
of the Environment; WQI: Water Quality Index. Table 3.
Figure 4. Groundwater quality heatmap
Volume 22 Issue 1 (2025) 139 doi: 10.36922/ajwep.8142