Page 87 - ESAM-1-4
P. 87
Engineering Science in
Additive Manufacturing TwinPrint: Dual-arm robotic bioprinting
a multi-material printing process (Video S1; video memory. Overall, the resolution was good enough for the
description is given in the “Supplemental information” application, as bioinks are of low viscosity and tend to
section in this article). fill up gaps after deposition. Hence, the inaccuracy is less
During printing, layer allocation and start point visible as compared to the pen ink test.
accuracy were observed. R1 was expected to print the 3.5. Formation of 3D multi-cellular disease model
bottom layer and retreat to “home,” avoiding collision with
R2 as it printed the next layer. This process was repeated In the context of an intact organism, cells inhabit a
until layer 7. It was noted that R2 would shift slightly when complex 3D environment, wherein cell-cell interaction
39
returning to the desired start point in subsequent layers. plays a pivotal role in tissue physiology and development.
This impacted the construct fidelity but was negligible Furthermore, the development of biomimicry disease
for small constructs. It is presumed that the inaccuracy models necessitates the precise replication of diverse
is due to mechanical constraints and the robot’s cache cellular interactions, as this process is imperative in the
formation of the diseased tissue microenvironment. 40,41 To
A B test our system’s potential for developing a multi-cellular
disease model, we performed printing using two types of
cells; AML cell line (HL60) and human BM-MSCs in a ratio
of 3:1. Human BM-MSCs are well established as accessory
cells that confer survival signals and chemoresistance
advantage to acute leukemia cells. Regarding bioink, we
42
Figure 7. Acellular 3D construct of the peptide bioink IVZK with one used IVZK peptide-based bioink. 19,20
bioink printing batch in green color (for one robotic arm) and another in
clear color (for the second robotic arm) assembled in an alternating layer Our results demonstrated the potential of our dual-
approach. (A) Side view; (B) top view. arm robotic system for the controlled deposition of
A B
C D
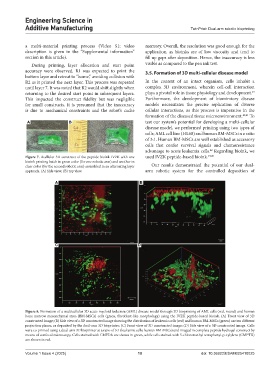
Figure 8. Formation of a multicellular 3D acute myeloid leukemia (AML) disease model through 3D bioprinting of AML cells (red, round) and human
bone marrow mesenchymal stem (BM-MSCs) cells (green, fibroblast-like morphology) using the IVZK peptide-based bioink. (A) Front view of 3D
constructed image; (B) Side view of a 3D constructed image showing the distribution of leukemia cells (red) and human BM-MSCs (green) on two different
projection planes, as deposited by the dual-arm 3D bioprinter; (C) Front view of 3D constructed image; (D) Side view of a 3D constructed image. Cells
were co-printed using a dual-arm 3D bioprinter at a ratio of 3:1 (leukemia cells: human BM-MSCs) and imaged in complete peptide hydrogel construct by
means of confocal microscopy. Cells stained with CMFDA are shown in green, while cells stained with 5-chloromethyl tetraphenyl-p-xylylene (CMPTX)
are shown in red.
Volume 1 Issue 4 (2025) 10 doi: 10.36922/ESAM025410025