Page 88 - ESAM-1-4
P. 88
Engineering Science in
Additive Manufacturing TwinPrint: Dual-arm robotic bioprinting
A B
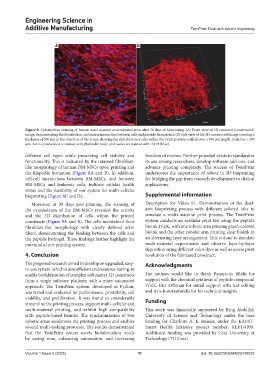
Figure 9. Cytoskeleton staining of human bone marrow mesenchymal stem after 30 days of bioprinting. (A) Front view of 3D rendered (constructed)
image, demonstrating the distribution and matrix interaction between cells and peptide biomaterial; (B) Side view of the 3D constructed image covering a
thickness of 500 µm in the direction of the Z axis, showing the distribution of cells within the IVZK peptide scaffold over a 500 µm length. Scale bar = 100
µm. Actin cytoskeleton is stained with phalloidin (red), and nuclei are stained with DAPI (blue).
different cell types while preserving cell viability and freedom of motion. Further potential exists to standardize
functionality. This is indicated by the retained fibroblast- its use among researchers, develop software add-ons, and
like morphology of human BM-MSCs upon printing and advance printing complexity. The success of TwinPrint
the filopodia formation (Figure 8A and B). In addition, underscores the importance of robots in 3D bioprinting
cell-cell interactions between BM-MSCs, and between for bridging the gap from research development to clinical
BM-MSCs and leukemic cells, indicate cellular health applications.
status and the feasibility of our system for multi-cellular
bioprinting (Figure 8C and D). Supplemental information
Moreover, at 30 days post-printing, the staining of Description for Video S1. Demonstration of the dual-
the cytoskeleton of the BM-MSCs revealed the activity arm bioprinting process with different colored inks to
and the 3D distribution of cells within the printed simulate a multi-material print process. The TwinPrint
constructs (Figure 9A and B). The cells maintained their system conducts an acellular print test using the peptide
fibroblast-like morphology with clearly defined actin bioink IVZK, with one robotic arm printing green-colored
fibers, demonstrating the binding between the cells and bioink and the other robotic arm printing clear bioink in
the peptide hydrogel. These findings further highlight the an alternating layer arrangement. This is done to simulate
potential of our printing system. multi-material experiments and observe layer-by-layer
deposition using different color dyes as well as assess print
4. Conclusion resolution of the fabricated construct.
The proposed research aimed to develop an upgraded, easy- Acknowledgments
to-use system, which is also efficient and resource-saving, to
enable biofabrication of complex soft matter 3D constructs The authors would like to thank Panayiotis Bilalis for
from a single software platform with a more automated support with the chemical synthesis of peptide compound
approach. The TwinPrint system, developed in Python, IVZK, Eter Othman for initial support with text editing
was tested and evaluated for performance, printability, cell and Aris Konstantinidis for his technical insights.
viability, and proliferation. It was found to considerably Funding
streamline the printing process, support multi-cellular and
multi-material printing, and exhibit high compatibility This work was financially supported by King Abdullah
with peptide-based bioinks. The synchronization of two University of Science and Technology under the base
robotic arms accelerates the printing process and enables funding for Charlotte A. E. Hauser, under the KAUST-
several multi-tasking protocols. The results demonstrated Smart Health Initiative project number: REI/1/4938.
that the TwinPrint system meets biofabrication needs Additional funding was provided by Graz University of
by saving time, enhancing automation, and increasing Technology (TU Graz).
Volume 1 Issue 4 (2025) 11 doi: 10.36922/ESAM025410025