Page 59 - GHES-1-2
P. 59
Global Health Econ Sustain Antimicrobial eco-friendly material
Table 2. The obtained critical pH points, including
additional mathematical parameters from Boltzmann fitting
Sample Critical pH R 2 Chi‑square
BP-g-4VP (7.2±0.9 g%) 11.0 0.95 1.10
BP-g-4VP (11.7±0.6 g%%) 6.8 0.97 1.06
BP-g-4VP (18.8±0.9 g%) 5.5 0.96 1.49
BP-g-4VP (22.7±0.5 g%) 5.8 0.95 1.96
BP-g-4VP (43.4±1.7 g%) 6.0 0.97 1.61
BP-g-4VP (56.0±2.4 g%) 8.0 0.95 7.39
BP-g-4VP (64.9±1.1 g%) 7.4 0.94 2.82
Table 3. Decomposition temperatures (TD) of BP, poly
(4VP), BP‑g‑4VP, and VR‑g‑4VP Ag
@
T (°C) BP Poly BP‑g‑4VP BP‑g‑4VP
(4VP) (31.6 g%) (31.6 g%) Ag
@
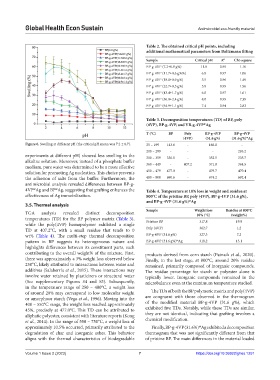
Figure 6. Swelling at different pH (the critical pH mean was 7.2 ± 0.7). 25 – 199 143.6 - 166.8 -
200 – 299 - - - 293.2
experiments at different pH) showed less swelling in the 300 – 359 330.5 - 352.5 335.7
alkaline solution. Moreover, instead of a phosphate buffer
medium, pure water was determined to be a more effective 360 – 449 - 407.2 371.8 364.5
solution for promoting Ag nucleation. This choice prevents 450 – 479 477.0 - 479.7 479.4
the adhesion of salts from the buffer. Furthermore, the 480 – 800 690.6 - 674.2 692.4
antimicrobial analysis revealed differences between BP-g-
4VP Ag and BP Ag, suggesting that grafting enhances the Table 4. Temperature at 10% loss in weight and residue at
@
@
effectiveness of Ag immobilization. 800°C of the pristine BP, poly (4VP), BP‑g‑4VP (31.6 g%),
and BP‑g‑4VP (31.6 g%) Ag
@
3.5. Thermal analysis
Sample Weight loss Residue at 800°C
TGA analysis revealed distinct decomposition 10% (°C) (weight%)
temperatures (TD) for the BP polymer matrix (Table 3), Pristine BP 317.8 19.9
while the poly(4VP) homopolymer exhibited a single
TD at 407.2°C, with a small residue that tends to 0 Poly (4VP) 362.7 1.2
wt% (Table 4). The multi-step thermal decomposition BP-g-4VP (31.6 g%) 327.3 7.2
pattern in BP suggests its heterogeneous nature and BP-g-4VP (31.6 g%) Ag 318.2 13.1
@
highlights differences between its constituent parts, each
contributing to the overall weight% of the mixture. First, products derived from corn starch (Patnaik et al., 2020).
there was approximately a 3% weight loss observed below Finally, in the last stage, at 800°C, around 20% residue
250°C, likely attributed to interactions between water and remained, primarily composed of inorganic compounds.
additives (Salaberria et al., 2015). These interactions may The residue percentage for starch or polyester alone is
involve water retained by plasticizers or structural water typically lower. Inorganic compounds remained in the
(See supplementary Figures S4 and S5). Subsequently, microbalance even at the maximum temperature studied.
in the temperature range of 250 – 400°C, a weight loss
of around 20% may correspond to low molecular weight The TDs of both the BP polymeric matrix and poly(4VP)
or amorphous starch (Vega et al., 1996). Moving into the are congruent with those observed in the thermogram
400 – 550°C range, the weight loss reached approximately of the modified material BP-g-4VP (31.6 g%), which
45%, precisely at 477.0°C. This TD can be attributed to exhibited five TDs. Notably, while these TDs are similar,
aliphatic polyester, consistent with literature reports (Kong they are not identical, indicating that grafting involves a
et al., 2014). In the range of 550 – 750°C, a weight loss of chemical modification.
approximately 10.5% occurred, primarily attributed to the Finally, BP-g-4VP (31.6%) Ag exhibited a decomposition
@
degradation of char and inorganic ashes. This behavior thermogram that was not significantly different from that
aligns with the thermal characteristics of biodegradable of pristine BP. The main differences in the material loaded
Volume 1 Issue 2 (2023) 7 https://doi.org/10.36922/ghes.1251