Page 336 - IJB-10-1
P. 336
International Journal of Bioprinting 3D-printed bone scaffolds and biofilm formation
Cucamonga, California, USA) for 5 min, and then allowed the samples were washed using PBS and then fixed for
to air-dry in a 24-well plate. A wound isolate of S. aureus 2 h using 2% glutaraldehyde at 4°C. The samples were
40
strain ATCC-29213 was chosen as the source of medical then dehydrated in a series of ethanol of different EtOH
implant bacterial infection to study its effectiveness in concentrations, specifically in the following order: 25%,
the in vitro formation of biofilm in biomaterials. In 50%, 75%, 90%, 100%, 50% ethanol/hexamethyldisilazane
37
accordance with a published study, an overnight culture (Sigma-Aldrich, St. Louis, Missouri, USA), and 100%
of S. aureus was diluted 1:100 in Tryptic Soy Broth (TSB) hexamethyldisilazane. The next day, the samples were
purchased from Corning (Corning, New York, USA) examined with SEM as described in section 2.4.1.
before being transferred to the samples in 24-well plate
for a 48-h incubation at 37°C (the media was aspirated, 2.4. Scaffold characterization
and fresh TSB was supplied after 24 h). The bacterial
38
suspension was removed, and the wells were washed twice 2.4.1. Pore size and morphological analysis
with sterile distilled water. To fix the biofilm, plates were SEM was performed using TeScan Vega (TeScan, Brno, Czech
incubated at 60°C for 1 h before staining with crystal violet Republic). To improve the polymeric scaffold’s conductivity
(0.1% w/v) acquired from Loba Chemie Pvt. Ltd. (Colaba, and avoid charging effects, a thin layer of gold was deposited
Mumbai, India). The plates were washed twice with by sputtering using a JFC-1600 (JEOL, Tokyo, Japan) auto-
39
sterile phosphate-buffered saline (PBS; Invitrogen, Thermo fine coater for 60 s under 7 Pa pressure. SEM images were
Fisher Scientific, Waltham, Massachusetts, USA) and then used to measure the actual scaffold pore size and analyze the
left to dry. Ethanol (70% v/v) was added, and the plates morphology of the biofilm formation. Three measurements
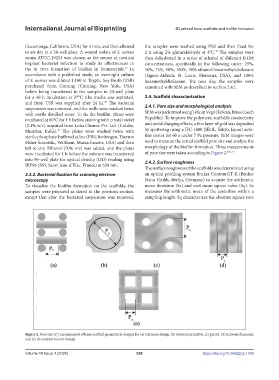
were incubated for 1 h before the mixture was transferred of pore size were taken according to Figure 2. 8,21,41
into 96-well plate for optical density (OD) reading using 2.4.2. Surface roughness
IRE96 (Sfri, Saint-Jean-d’Illac, France) at 630 nm.
The surface roughness of the scaffolds was determined using
2.3.2. Bacterial fixation for scanning electron an optical profiling system Bruker ContourGT-K (Bruker
microscopy Nano Gmbh, Berlin, Germany) to acquire the arithmetic
To visualize the biofilm formation on the scaffolds, the mean deviation (Sa) and root mean square value (Sq). Sa
samples were prepared as stated in the previous section, measures the arithmetic mean of the centerline within a
except that after the bacterial suspension was removed, sampling length. Sq characterizes the absolute square root
Figure 2. Pore size (P ) measurement of bone scaffold geometrical designs for (a) reference design, (b) Schwarz primitive, (c) gyroid, (d) Schwarz diamond,
s
and (e) Re-entrant auxetic design.
Volume 10 Issue 1 (2024) 328 https://doi.org/10.36922/ijb.1768