Page 398 - IJB-10-1
P. 398
International Journal of Bioprinting In situ bioprinting for cartilage repair
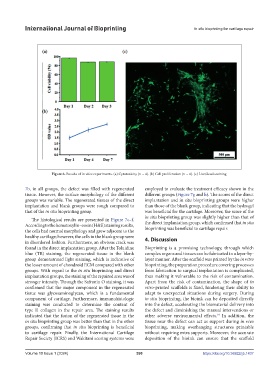
Figure 6. Results of in vitro experiments. (a) Cytotoxicity (n = 4). (b) Cell proliferation (n = 4). (c) Live/dead staining.
7b, in all groups, the defect was filled with regenerated employed to evaluate the treatment efficacy shown in the
tissue. However, the surface morphology of the different different groups (Figure 7g and h). The scores of the direct
groups was variable. The regenerated tissues of the direct implantation and in situ bioprinting groups were higher
implantation and blank groups were rough compared to than those of the blank group, indicating that the hydrogel
that of the in situ bioprinting group. was beneficial for the cartilage. Moreover, the score of the
The histological results are presented in Figure 7c–f. in situ bioprinting group was slightly higher than that of
According to the hematoxylin–eosin (H&E) staining results, the direct implantation group, which confirmed that in situ
the cells had normal morphology and grew adjacent to the bioprinting was beneficial to cartilage repair.
healthy cartilage; however, the cells in the blank group were 4. Discussion
in disordered fashion. Furthermore, an obvious crack was
found in the direct implantation group. After the Toluidine Bioprinting is a promising technology, through which
blue (TB) staining, the regenerated tissue in the blank complex organs and tissues can be fabricated in a layer-by-
group demonstrated light staining, which is indicative of layer manner. After the scaffold was printed by the in vitro
the lesser amount of chondroid ECM compared with other bioprinting, the preparation procedure covering processes
groups. With regard to the in situ bioprinting and direct from fabrication to surgical implantation is complicated,
implantation groups, the staining of the repaired area was of thus making it vulnerable to the risk of contamination.
stronger intensity. Through the Safranin O staining, it was Apart from the risk of contamination, the shape of in
confirmed that the major component in the regenerated vitro-printed scaffolds is fixed, hindering their ability to
tissue was glycosaminoglycan, which is a fundamental adapt to unexpected situations during surgery. During
component of cartilage. Furthermore, immunohistologic in situ bioprinting, the bioink can be deposited directly
staining was conducted to determine the content of into the defect, accelerating the biomaterial delivery into
type II collagen in the repair area. The staining results the defect and diminishing the manual interventions or
indicated that the fusion of the regenerated tissue in the other adverse environmental effects. In addition, the
19
in situ bioprinting group was better than that in the other tissue near the defect can act as support during in vivo
groups, confirming that in situ bioprinting is beneficial bioprinting, making overhanging structures printable
to cartilage repair. Finally, the International Cartilage without requiring extra supports. Moreover, the accurate
Repair Society (ICRS) and Wakitani scoring systems were deposition of the bioink can ensure that the scaffold
Volume 10 Issue 1 (2024) 390 https://doi.org/10.36922/ijb.1437