Page 53 - IJB-2-1
P. 53
Elizabeth V. Koudan, Elena A. Bulanova, Frederico DAS Pereira, et al
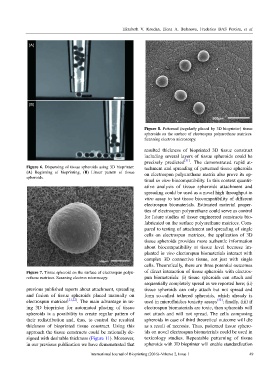
Figure 8. Patterned (regularly placed by 3D bioprinter) tissue
spheroids on the surface of electrospun polyurethane matrices.
Scanning electron microscopy.
resulted thickness of bioprinted 3D tissue construct
including several layers of tissue spheroids could be
[31]
precisely predicted . The demonstrated rapid at-
Figure 6. Dispensing of tissue spheroids using 3D bioprinter: tachment and spreading of patterned tissue spheroids
(A) Beginning of bioprinting, (B) Linear pattern of tissue on electrospun polyurethane matrix also prove its op-
spheroids.
timal in vitro biocompatibility. In this context quantit-
ative analysis of tissue spheroids attachment and
spreading could be used as a novel high throughput in
vitro assay to test tissue biocompatibility of different
electrospun biomaterials. Estimated material proper-
ties of electrospun polyurethane could serve as control
for future studies of tissue engineered constructs bio-
fabricated on the surface polyurethane matrices. Com-
pared to testing of attachment and spreading of single
cells on electrospun matrices, the application of 3D
tissue spheroids provides more authentic information
about biocompatibility at tissue level because im-
planted in vivo electorspun biomaterials interact with
complex 3D connective tissue, not just with single
cells. Theoretically, there are three potential outcomes
Figure 7. Tissue spheroid on the surface of electrospun polyu- of direct interaction of tissue spheroids with electros-
rethane matrices. Scanning electron microscopy. pun biomaterials: (i) tissue spheroids can attach and
sequentially completely spread as we reported here; (ii)
previous published reports about attachment, spreading tissue spheroids can only attach but not spread and
and fusion of tissue spheroids placed manually on form so-called tethered spheroids, which already is
electrospun matrices [21,22] . The main advantage in us- used in microfluidics toxicity assays [32] ; finally, (iii) if
ing 3D bioprinter for automated placing of tissue electrospun biomaterials are toxic, then spheroids will
spheroids is a possibility to create regular pattern of not attach and will not spread. The cells composing
their redistribution and, thus, to control the resulted spheroids in case of third theoretical outcome will die
thickness of bioprinted tissue construct. Using this as a result of necrosis. Thus, patterned tissue sphero-
approach the tissue constructs could be rationally de- ids on novel electrospun biomaterials could be used in
signed with desirable thickness (Figure 11). Moreover, toxicology studies. Repeatable patterning of tissue
in our previous publication we have demonstrated that spheroids with 3D bioprinter will enable standardization
International Journal of Bioprinting (2016)–Volume 2, Issue 1 49