Page 545 - IJB-10-2
P. 545
International Journal of Bioprinting Bottom-up and top-down VAT photopolimerization
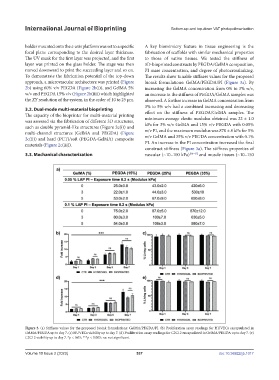
holder mounted onto the z-axis platform was set to a specific A key biomimicry feature in tissue engineering is the
focal plane corresponding to the desired layer thickness. fabrication of scaffolds with similar mechanical properties
The UV mask for the first layer was projected, and the first to those of native tissues. We tested the stiffness of
layer was printed on the glass holder. The stage was then 3D-bioprinted constructs by PEGDA/GelMA composition,
moved downward to print the succeeding layer and so on. PI mass concentration, and degree of photocrosslinking.
To demonstrate the fabrication potential of the top-down The results show tunable stiffness values for the proposed
approach, a microvascular architecture was printed (Figure bioink formulations: GelMA/PEGDA/PI (Figure 3a). By
2b) using 60% v/v PEGDA (Figure 2b(i)), and GelMA 5% increasing the GelMA concentration from 0% to 3% w/v,
w/v and PEGDA 15% v/v (Figure 2b(iii)) which highlighted an increase in the stiffness of PEGDA/GelMA samples was
the XY resolution of the system, in the order of 10 to 25 µm. observed. A further increase in GelMA concentration from
3% to 5% w/v had a combined increasing and decreasing
3.2. Dual-mode multi-material bioprinting effect on the stiffness of PEGDA/GelMA samples. The
The capacity of the bioprinter for multi-material printing
was assessed via the fabrication of different 3D structures, minimum average elastic modulus obtained was 22 ± 1.0
such as double pyramid-like structures (Figure 2c(i)) and kPa for 3% w/v GelMA and 15% v/v PEGDA with 0.05%
multi-channel structures (GelMA and PEGDA) (Figure w/v PI, and the maximum modulus was 870 ± 8 kPa for 5%
2c(ii)) and hard (PCT)/soft (PEGDA-GelMA) composite w/v GelMA and 35% v/v PEGDA concentration with 0.1%
materials (Figure 2c(iii)). PI. An increase in the PI concentration increased the final
construct stiffness (Figure 3a). The stiffness properties of
3.3. Mechanical characterization vascular (~10–100 kPa) [46-47] and muscle tissues (~10–150
Figure 3. (a) Stiffness values for the proposed bioink formulations: GelMA/PEGDA/PI. (b) Proliferation assay readings for HUVECs encapsulated in
GelMA/PEGDA up to day 7. (c) HUVECs viability up to day 7. (d) Proliferation assay readings for C2C12 encapsulated in GelMA/PEGDA up to day 7. (e)
C2C12 viability up to day 7. *p < 0.05; ***p < 0.005; ns: not significant.
Volume 10 Issue 2 (2023) 537 doi: 10.36922/ijb.1017