Page 47 - IJB-3-1
P. 47
Lothar Koch, Ole Brandt, Andrea Deiwick, et al.
bubble expands by vapor pressure and propels the ad-
jacent biomaterial forward, which then is deposited as
a droplet at a predefined position on a collector slide.
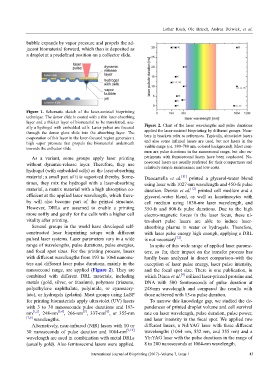
Figure 1. Schematic sketch of the laser-assisted bioprinting
technique. The donor slide is coated with a thin laser-absorbing
layer and a thicker layer of biomaterial to be transferred, usu-
ally a hydrogel with embedded cells. Laser pulses are focused Figure 2. Chart of the laser wavelengths and pulse durations
through the donor glass slide into the absorbing layer. The applied for laser-assisted bioprinting by different groups. Num-
evaporation of this layer in the laser-focused region generates a bers in brackets refer to references. Typically, ultraviolet lasers
high vapor pressure that propels the biomaterial underneath and also some infrared lasers are used, but not lasers in the
towards the collector slide. visible range (ca. 380–780 nm, colored background). Most com-
mon are pulse durations in the nanosecond range, but also ex-
As a variant, some groups apply laser printing periments with femtosecond lasers have been conducted. Na-
without dynamic-release layer. Therefore, they use nosecond lasers are usually preferred for their compactness and
relatively simple maintenance and low costs.
hydrogel (with embedded cells) as the laser-absorbing
material; a small part of it is vaporized thereby. Some- Duocastella et al. [11] printed a glycerol-water blend
times, they mix the hydrogel with a laser-absorbing using laser with 1027-nm wavelength and 450-fs pulse
material, a matrix material with a high absorption co- duration. Desrus et al. [12] printed cell medium and a
efficient at the applied laser wavelength, which there- glycerol-water blend, as well as keratinocytes with
by will also become part of the printed structure. cell medium using 1030-nm laser wavelength, and
However, DRLs are assumed to enable a printing 350-fs and 800-fs pulse durations. Due to the high
more softly and gently for the cells with a higher cell electro-magnetic forces in the laser focus, these ul-
vitality after printing. tra-short pulse lasers are able to induce laser-
Several groups in the world have developed self- absorbing plasma in water or hydrogels. Therefore,
constructed laser bioprinting setups with different with laser pulse energy high enough, applying a DRL
pulsed laser systems. Laser parameters vary in a wide is not necessary [12] .
range of wavelengths, pulse durations, pulse energies, In spite of this wide range of applied laser parame-
and focal spot sizes. For the printing process, lasers ters, so far, their impact on the transfer process has
with different wavelengths from 193 to 1064 nanome- hardly been analyzed in direct comparison–with the
ters and different laser pulse durations, mainly in the exception of laser pulse energy, laser pulse intensity,
nanosecond range, are applied (Figure 2). They are and the focal spot size. There is one publication, in
[4]
combined with different DRL materials, including which Dinca et al. utilized laser-printed proteins and
metals (gold, silver, or titanium), polymers (triazene, DNA with 500 femtoseconds of pulse duration at
polyethylene naphthalate, polyimide, or cyanoacry- 248-nm wavelength and compared the results with
late), or hydrogels (gelatin). Most groups using LaBP those achieved with 15-ns pulse duration.
for printing biomaterials apply ultraviolet (UV) lasers To narrow this knowledge gap, we studied the de-
with 3 to 30 nanoseconds pulse durations and 193- pendences of printed droplet volume and cell survival
[6]
[5]
nm [1,2] , 248-nm [3,4] , 266-nm , 337-nm , or 355-nm rate on laser wavelength, pulse duration, pulse power,
[7,8] wavelengths. and laser intensity in the focal spot. We applied two
Alternatively, near-infrared (NIR) lasers with 10 or different lasers, a Nd:YAG laser with three different
30 nanoseconds of pulse duration and 1064-nm [9,10] wavelengths (1064 nm, 532 nm, and 355 nm) and a
wavelength are used in combination with metal DRLs Yb:YAG laser with the pulse durations in the range of
(usually gold). Also femtosecond lasers were applied. 8 to 200 nanoseconds at 1064-nm wavelength.
International Journal of Bioprinting (2017)–Volume 3, Issue 1 43