Page 277 - IJB-10-3
P. 277
International Journal of Bioprinting 3D bioscaffolds with SR1 for vasculogenesis
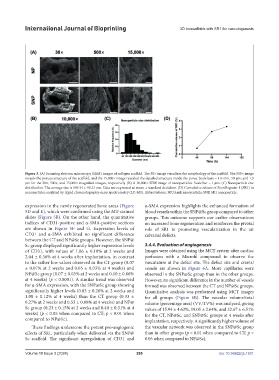
Figure 3. (A) Scanning electron microscopy (SEM) images of collagen scaffold. The 30× image visualizes the morphology of the scaffold. The 500× image
reveals the porous structure of the scaffold, and the 15,000× image revealed the detailed structure inside the pores. Scale bars = 1.0 mm, 50 µm, and 1.5
µm for the 30×, 500×, and 15,000× magnified images, respectively. (B) A 10,000× SEM image of nanoparticles. Scale bar = 1 µm. (C) Nanoparticle size
distribution. The average size is 189.34 ± 99.23 nm. Data are expressed as mean ± standard deviation. (D) Cumulative release of StemRegenin-1 (SR1) in
nanoparticles analyzed by liquid chromatography-mass spectrometry (LC-MS). Abbreviations: NP, blank nanoparticle; SNP, SR1 nanoparticle.
expression in the newly regenerated bone areas (Figure α-SMA expression highlights the enhanced formation of
5D and E), which were confirmed using the MT-stained blood vessels within the SNP@Sc group compared to other
slides (Figure 5B). On the other hand, the quantitative groups. This outcome supports our earlier observations
indices of CD31-positive and α-SMA-positive sections on increased bone regeneration and reinforces the pivotal
are shown in Figure 5F and G. Expression levels of role of SR1 in promoting vascularization in the rat
CD31 and α-SMA exhibited no significant difference calvarial defects.
between the CT and NP@Sc groups. However, the SNP@
Sc group displayed significantly higher expression levels 3.4.4. Evaluation of angiogenesis
of CD31, with values of 1.06 ± 0.18% at 2 weeks and Images were obtained using the MCT system after cardiac
2.04 ± 0.36% at 4 weeks after implantation, in contrast perfusion with a Microfil compound to observe the
to the rather low values observed in the CT group (0.07 vasculature at the defect site. The defect site and cranial
± 0.07% at 2 weeks and 0.05 ± 0.03% at 4 weeks) and vessels are shown in Figure 6A. More capillaries were
NP@Sc group (0.07 ± 0.03% at 2 weeks and 0.10 ± 0.06% observed in the SNP@Sc group than in the other groups.
at 4 weeks) (p < 0.0001). A similar trend was observed However, no significant difference in the number of vessels
for α-SMA expression, with the SNP@Sc group showing formed was observed between the CT and NP@Sc groups.
significantly higher levels (0.83 ± 0.26% at 2 weeks and Quantitative analysis was performed using MCT images
1.08 ± 0.12% at 4 weeks) than the CT group (0.33 ± for all groups (Figure 6B). The vascular volume/total
0.27% at 2 weeks and 0.53 ± 0.08% at 4 weeks) and NP@ volume (percentage area) (VV/TV%) was analyzed, giving
Sc group (0.23 ± 0.15% at 2 weeks and 0.40 ± 0.31% at 4 values of 15.94 ± 4.62%, 19.05 ± 2.64%, and 32.67 ± 6.51%
weeks) (p < 0.05 when compared to CT; p < 0.01 when for the CT, NP@Sc, and SNP@Sc groups at 4 weeks after
compared to NP@Sc). implantation, respectively. A significantly higher volume of
These findings underscore the potent pro-angiogenic the vascular network was observed in the SNP@Sc group
effects of SR1, particularly when delivered via the SNP@ than in other groups (p < 0.01 when compared to CT; p <
Sc scaffold. The significant upregulation of CD31 and 0.05 when compared to NP@Sc).
Volume 10 Issue 3 (2024) 269 doi: 10.36922/ijb.1931