Page 280 - IJB-10-3
P. 280
International Journal of Bioprinting 3D bioscaffolds with SR1 for vasculogenesis
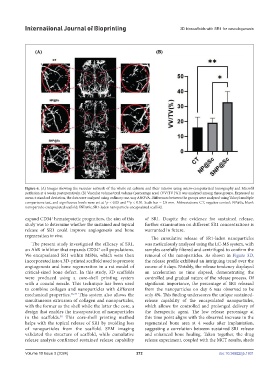
Figure 6. (A) Images showing the vascular network of the whole rat calvaria and their interior using micro-computerized tomography and Microfil
perfusion at 4 weeks postoperatively. (B) Vascular volume/total volume (percentage area) (VV/TV [%]) was analyzed among three groups. Expressed as
mean ± standard deviation, the data were analyzed using ordinary one-way ANOVA. Differences between the groups were analyzed using Tukey’s multiple
comparisons test, and significance levels were set at *p < 0.05 and **p < 0.01. Scale bar = 2.0 mm. Abbreviations: CT, negative control; NP@Sc, blank
nanoparticle-encapsulated scaffold; SNP@Sc, SR1-laden nanoparticle-encapsulated scaffold.
expand CD34 hematopoietic progenitors, the aim of this of SR1. Despite the evidence for sustained release,
+
study was to determine whether the sustained and topical further examination on different SR1 concentrations is
release of SR1 could improve angiogenesis and bone warranted in future.
regeneration in vivo.
The cumulative release of SR1-laden nanoparticles
The present study investigated the efficacy of SR1, was meticulously analyzed using the LC-MS system, with
an AhR inhibitor that expands CD34 cell populations. samples carefully filtered and centrifuged to confirm the
+
We encapsulated SR1 within MSNs, which were then removal of the nanoparticles. As shown in Figure 3D,
incorporated into a 3D-printed scaffold used to promote the release profile exhibited an intriguing trend over the
angiogenesis and bone regeneration in a rat model of course of 6 days. Notably, the release tendency displayed
critical-sized bone defect. In this study, 3D scaffolds an acceleration as time elapsed, demonstrating the
were produced using a core-shell printing system controlled and gradual nature of the release process. Of
with a coaxial nozzle. This technique has been used significant importance, the percentage of SR1 released
to combine collagen and nanoparticles with different from the nanoparticles on day 6 was observed to be
mechanical properties. 36,37 This system also allows the only 4%. This finding underscores the unique sustained-
simultaneous extrusion of collagen and nanoparticles, release capability of the encapsulated nanoparticles,
with the former as the shell while the latter the core, a which allows for controlled and prolonged delivery of
design that enables the incorporation of nanoparticles the therapeutic agent. The low release percentage at
in the scaffolds. This core-shell printing method this time point aligns with the observed increase in the
38
helps with the topical release of SR1 by avoiding loss regenerated bone area at 4 weeks after implantation,
of nanoparticles from the scaffold. SEM imaging suggesting a correlation between sustained SR1 release
validated the structure of scaffold, while cumulative and enhanced bone healing. Taken together, the drug
release analysis confirmed sustained release capability release experiment, coupled with the MCT results, sheds
Volume 10 Issue 3 (2024) 272 doi: 10.36922/ijb.1931