Page 61 - IJB-5-2
P. 61
Shuai C, et al.
On the other hand, the rapid solidification occurred in solubility of Al in α-Mg is about 12.7 wt% at the eutectic
SLM also played a key role in the formation of continuous temperature of 437°C and its solid solubility at room
net-like eutectic α phase. According to the Mg-Al binary temperature is about 2 wt%. In the present study, SLM,
equilibrium phase diagram , the maximum solid which involved a fast cooling rate, was applied to fabricate
[29]
AZ61-Ti alloy. Moreover, such a rapid solidification was
believed to promote the occurrence of eutectic reaction
at lower eutectic temperature and critical hypoeutectic
Al content (as compared with equilibrium). The eutectic
reaction precipitated the eutectic α phase and eutectic β
particles, which would attach to the primary α-Mg grains
and distribute in grain boundaries, respectively. Moreover,
the fast cooling rate was conducive for the homogeneous
precipitation of the eutectic α phase and eutectic β particles.
Therefore, the eutectic α phase distributed continuously
and formed a net-like structure along the grain boundaries.
Impacts of Ti on the grain sizes were also observed in this
study. In Ti containing Mg-Al alloys, Ti element served as
a surface active element, which could significantly reduce
the alloy solid-liquid interfacial tension and decrease the
nucleation energy during the solidification process .
[30]
According to the nucleation formula, r*=−2σ /∆G ;
[31]
m
LS
where r* was the critical nucleation radius, σ was the
LS
solid-liquid interfacial tension, and ∆G was the Gibbs
m
free energy of solidification. Ti reduced the solid-liquid
interfacial tension, thereby reducing the critical nucleation
radius. As a result, the ultimately nuclear volume of the
primary α-Mg was improved, thus forming refined grain
size. Furthermore, in AZ61-0.75Ti and AZ61-1.0Ti,
excessive Ti combined with Al to form the TiAl phase.
3
Although the TiAl phase was not regarded as the core
3
of crystal formation, it would be pushed to the front of
solid-liquid interfaces and prevented the grain growth.
Therefore, the grain sizes decreased continuously with
increasing Ti content.
4.2 Corrosion Behaviors
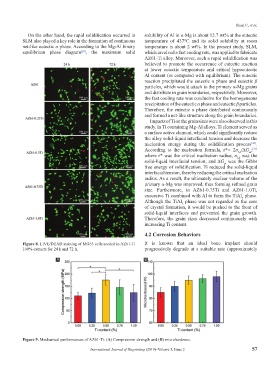
Figure 8. LIVE/DEAD staining of MG63 cells seeded in AZ61-Ti It is known that an ideal bone implant should
100% extracts for 24 h and 72 h. progressively degrade at a suitable rate (approximately
A B
Figure 9. Mechanical performances of AZ61-Ti: (A) Compression strength and (B) microhardness.
International Journal of Bioprinting (2019)–Volume 5, Issue 2 57