Page 83 - IJB-5-2
P. 83
Arab W, et al.
A
B C
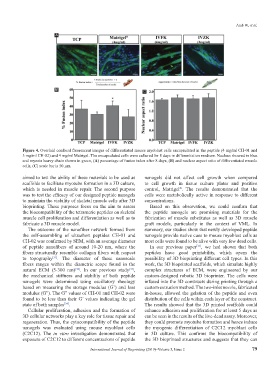
Figure 4. Overlaid confocal fluorescent images of differentiated mouse myoblast cells encapsulated in the peptide (4 mg/ml CH-01 and
3 mg/ml CH-02) and 4 mg/ml Matrigel. The encapsulated cells were cultured for 8 days in differentiation medium. Nucleus showed in blue
and myosin heavy chain shown in green, (A) percentage of fusion index after 8 days, (B) and nuclear aspect ratio of differentiated muscle
cells, (C) scale bar is 50 µm.
aimed to test the ability of these materials to be used as nanogels did not affect cell growth when compared
scaffolds to facilitate myotube formation in a 3D culture, to cell growth in tissue culture plates and positive
which is needed in muscle repair. The second purpose control, Matrigel . The results demonstrated that the
®
was to test the efficacy of our designed peptide nanogels cells were metabolically active in response to different
to maintain the viability of skeletal muscle cells after 3D concentrations.
bioprinting. These purposes focus on the aim to assess Based on this observation, we could confirm that
the biocompatibility of the tetrameric peptides on skeletal the peptide nanogels are promising materials for the
muscle cell proliferation and differentiation as well as to fabrication of muscle substitutes as well as 3D muscle
fabricate a 3D muscle model. graft models, particularly in the context of VML. In
The outcome of the nanofiber network formed from summary, our studies show that newly developed peptide
the self-assembling of ultrashort peptides CH-01 and nanogels provide native cues to mouse myoblast cells as
CH-02 was confirmed by SEM, with an average diameter most cells were found to be alive with very few dead cells.
of peptide nanofibers of around 10-20 nm, where the In our previous paper , we had shown that both
[33]
fibers structurally resemble collagen fibers with respect peptides have good printability, which opens the
to topography . The diameter of these nanoscale possibility of 3D bioprinting different cell types. In this
[31]
fibers ranges within the diametric scope found in the work, the 3D bioprinted scaffolds, which simulate highly
natural ECM (5-300 nm) . In our previous study , complex structures of ECM, were engineered by our
[33]
[32]
the mechanical stiffness and stability of both peptide custom-designed robotic 3D bioprinter. The cells were
nanogels were determined using oscillatory rheology infused into the 3D constructs during printing through a
based on measuring the storage modulus (G’) and loss custom extrusion method. The two-inlet nozzle, fabricated
modulus (G”). The G” values of CH-01 and CH-02 were in-house, allowed the gelation of the peptide and even
found to be less than their G’ values indicating the gel distribution of the cells within each layer of the construct.
state of both samples . The results showed that the 3D printed scaffolds could
[34]
Cellular proliferation, adhesion and the formation of enhance adhesion and proliferation for at least 5 days as
3D cellular networks play a key role for tissue repair and can be seen in the results of the live-dead assay. Moreover,
regeneration. Thus, the cytocompatibility of the peptide they could promote myotube formation and hence induce
nanogels was evaluated using mouse myoblast cells the myogenic differentiation of C2C12 myoblast cells
(C2C12). The in vitro investigation demonstrated that in 3D culture. This confirms the biocompatibility of
exposure of C2C12 to different concentrations of peptide the 3D bioprinted structures and suggests that they can
International Journal of Bioprinting (2019)–Volume 5, Issue 2 79