Page 84 - IJB-5-2
P. 84
Exploring nanofibrous self-assembling peptide hydrogels using mouse myoblast cells for three-dimensional bioprinting and tissue engineering applications
A D
B E
C F
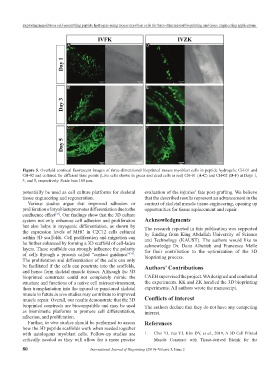
Figure 5. Overlaid confocal fluorescent images of three-dimensional bioprinted mouse myoblast cells in peptide hydrogels; CH-01 and
CH-02 and cultured for different time points (Live cells shown in green and dead cells in red) CH-01 (A-C) and CH-02 (D-F) at Days 1,
3, and 5, respectively. Scale bars 100 μm.
potentially be used as cell culture platforms for skeletal evaluation of the injuries’ fate post-grafting. We believe
tissue engineering and regeneration. that the described results represent an advancement in the
Various studies argue that improved adhesion or context of skeletal muscle tissue engineering, opening up
proliferation of myoblasts promotes differentiation due to the opportunities for tissue replacement and repair.
confluence effect . Our findings show that the 3D culture
[35]
system not only enhances cell adhesion and proliferation Acknowledgments
but also helps in myogenic differentiation, as shown by The research reported in this publication was supported
the expression levels of MHC in C2C12 cells cultured by funding from King Abdullah University of Science
within 3D scaffolds. Cell proliferation and migration can and Technology (KAUST). The authors would like to
be further enhanced by forming a 3D scaffold of cell-laden acknowledge Dr. Dana Alhattab and Francesca Melle
layers. These scaffolds can strongly influence the polarity for their contribution to the optimization of the 3D
of cells through a process called “contact guidance” . bioprinting process.
[35]
The proliferation and differentiation of the cells can only
be facilitated if the cells can penetrate into the scaffolds, Authors’ Contributions
and hence form skeletal muscle tissues. Although the 3D
bioprinted constructs could not completely mimic the CAEH supervised the project. WA designed and conducted
structure and functions of a native cell microenvironment, the experiments. KK and ZK handled the 3D bioprinting
their transplantation into the injured or punctured skeletal experiments. All authors wrote the manuscript.
muscle in future in vivo studies may contribute to improved
muscle repair. Overall, our results demonstrate that the 3D Conflicts of Interest
bioprinted constructs are biocompatible and may be used The authors declare that they do not have any competing
as biomimetic platforms to promote cell differentiation, interest.
adhesion, and proliferation.
Further, in vivo studies should be performed to assess References
how the 3D peptide scaffolds work when seeded together
with autologous myoblast cells. Follow-up studies are 1. Choi YJ, Jun YJ, Kim DY, et al., 2019, A 3D Cell Printed
critically needed as they will allow for a more precise Muscle Construct with Tissue-derived Bioink for the
80 International Journal of Bioprinting (2019)–Volume 5, Issue 2