Page 82 - IJB-5-2
P. 82
Exploring nanofibrous self-assembling peptide hydrogels using mouse myoblast cells for three-dimensional bioprinting and tissue engineering applications
A C
B D
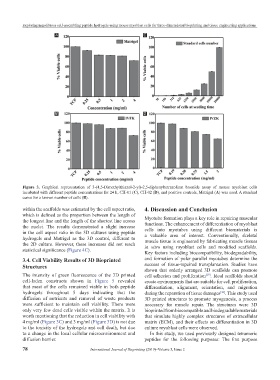
Figure 3. Graphical representation of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay of mouse myoblast cells
incubated with different peptide concentrations for 24 h, CH-01 (C), CH-02 (D), and positive controls, Matrigel (A) was used. A standard
curve for a known number of cells (B).
within the scaffolds was estimated by the cell aspect ratio, 4. Discussion and Conclusion
which is defined as the proportion between the length of
the longest line and the length of the shortest line across Myotube formation plays a key role in repairing muscular
functions. The enhancement of differentiation of myoblast
the nuclei. The results demonstrated a slight increase
in the cell aspect ratio in the 3D cultures using peptide cells into myotubes using different biomaterials is
a valuable area of interest. Conventionally, skeletal
hydrogels and Matrigel as the 3D control, different to muscle tissue is engineered by fabricating muscle tissues
the 2D culture. However, these increases did not reach in vitro using myoblast cells and modified scaffolds.
statistical significance (Figure 4C).
Key factors including biocompatibility, biodegradability,
3.4. Cell Viability Results of 3D Bioprinted and formation of polar parallel myotubes determine the
Structures success of tissue-repaired transplantation. Studies have
shown that orderly arranged 3D scaffolds can promote
The intensity of green fluorescence of the 3D printed cell adhesion and proliferation . Ideal scaffolds should
[29]
cell-laden constructs shown in Figure 5 revealed create environments that are suitable for cell proliferation,
that most of the cells remained viable in both peptide differentiation, alignment, orientation, and migration
hydrogels throughout 5 days indicating that the during the reparation of tissue damages . This study used
[30]
diffusion of nutrients and removal of waste products 3D printed structures to promote myogenesis, a process
were sufficient to maintain cell viability. There were necessary for muscle repair. The structures were 3D
only very few dead cells visible within the matrix. It is bioprinted from biocompatible and biodegradable materials
worth mentioning that the reduction in cell viability with that simulate highly complex structures of extracellular
4 mg/ml (Figure 3C) and 3 mg/ml (Figure 3D) is not due matrix (ECM), and their effects on differentiation in 3D
to the toxicity of the hydrogels and cell death, but due culture myoblast cells were observed.
to a change in the local cellular microenvironment and In this study, we used previously designed tetrameric
diffusion barrier. peptides for the following purposes: The first purpose
78 International Journal of Bioprinting (2019)–Volume 5, Issue 2