Page 147 - IJB-10-5
P. 147
International Journal of Bioprinting Liver printing: from structure to application
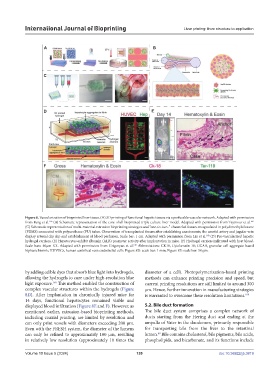
Figure 8. Vascularization of bioprinted liver tissue. (A) 3D printing of functional hepatic tissues via a perfusable vascular network. Adapted with permission
165
166
from Fang et al. (B) Schematic representation of the core–shell bioprinted triple culture liver model. Adapted with permission from Taymour et al.
(C) Schematic representation of multi-material extrusion bioprinting strategies and “one-to-two-” channeled tissues encapsulated in polydimethylsiloxane
(PDMS) connected with polyurethane (PU) tubes. Observation of transplanted tissues after establishing anastomosis; the carotid artery and jugular vein
160
display arterial clip slip and establishment of blood perfusion. Scale bar: 1 cm. Adapted with permission from Liu et al. (D) Pre-vascularized hepatic
hydrogel carriers. (E) Hepatocytes exhibit albumin (ALB) promoter activity after implantation in mice. (F) Hydrogel carriers infiltrated with host blood.
Scale bars: 40μm XX. Adapted with permission from Grigoryan et al. Abbreviations: CK18, Cytokeratin 18; GCAB, granular cell aggregate-based
146
biphasic bioink; HUVECs, human umbilical vein endothelial cells. Figure 8D: scale bar: 1 mm; Figure 8E: scale bar: 50 μm.
by adding edible dyes that absorb blue light into hydrogels, diameter of a cell). Photopolymerization-based printing
allowing the hydrogels to cure under high-resolution blue methods can enhance printing precision and speed, but
146
light exposure. This method enabled the construction of current printing resolutions are still limited to around 300
complex vascular structures within the hydrogels (Figure μm. Hence, further innovation in manufacturing strategies
8D). After implantation in chronically injured mice for is warranted to overcome these resolution limitations. 169
14 days, functional hepatocytes remained viable and
displayed blood infiltration (Figure 8E and F). However, as 5.2. Bile duct formation
mentioned earlier, extrusion-based bioprinting methods, The bile duct system comprises a complex network of
including coaxial printing, are limited by resolution and ducts starting from the Hering duct and ending at the
can only print vessels with diameters exceeding 200 μm. ampulla of Vater in the duodenum, primarily responsible
Even with the FRESH system, the diameter of the lumens for transporting bile from the liver to the intestinal
can only be refined to approximately 100 μm, resulting lumen. Bile contains cholesterol, bile pigments, bile acids,
40
in relatively low resolution (approximately 10 times the phospholipids, and bicarbonate, and its functions include
Volume 10 Issue 5 (2024) 139 doi: 10.36922/ijb.3819