Page 290 - IJB-10-5
P. 290
International Journal of Bioprinting 3D printing of collagen II-scaffolds
In contrast, on day 4 of culture (96 h), large cell aggregates
formed on all collagen II-based porous mesh scaffolds
(Figure 8D and E) rather than on their gelatin and collagen
I counterparts (Figure 8A and B), suggesting that collagen
II facilitates cell condensation. Cell distribution on each
material-based scaffold (on day 4) was further observed
under higher magnification. At high cell densities, cell
aggregates were absent in gelatin- and collagen I-based
scaffolds, highlighting that collagen II and the mesh
scaffold structure were crucial factors inducing aggregation
(Figure 9).
Furthermore, the OD value increased over time in all
scaffolds (Figure 9A). Within collagen II scaffolds, the
level of proliferation was significantly higher in sample d,
evidenced by the greater scaffold resolution (Figure 9B).
The proliferation rate of sample c (nonporous bulk
scaffolds) was significantly lower than that of samples d
and e (3D-printed porous mesh scaffolds) (Figure 9B).
For the expression of related genes, the level of
chondrogenic differentiation of MSCs corresponds to the
upregulation of chondrogenic markers. Chondrogenic
differentiation was higher in 3D-printed collagen II-based
mesh porous scaffolds than in their collagen I and gelatin
counterparts. The expression of chondrogenic markers
Figure 4. Creep strain of hydrogel inks (A) under a constant pressure was lower in the bulk nonporous collagen II-based scaffold
of 100 Pa and (B) with pressure removed. Abbreviation: CNF, cellulose (sample c) than in mesh porous scaffolds (Figure 10).
nanofiber. Collagen II-based mesh porous scaffolds with smaller
pores and thinner rods further enhanced chondrogenic
after cell culture (Figure 8; Table 2). Sample c, being a bulk differentiation, while osteogenic genes (COL1 and
nonporous sample, was not relevant to the discussion on RUNX2) were upregulated in collagen I-based scaffolds.
rod diameter and pore size changes. According to our Therefore, the compositional and structural characteristics
results, the swelling was minimal, with observed shrinkage. of the scaffolds were optimized simultaneously for sample
The minimal dimensional change after rehydration could d to enhance chondrogenic differentiation. The expression
be contributed by the rigid nanoscale network of CNF and of the hypoxic factor HIF-1α displayed a similar trend for
the high concentration of the crosslinking solution. cell proliferation and was the highest in samples b and
d. Lastly, high scaffold resolution significantly facilitated
3.4. Biological study cell condensation, observed from the significantly
At 12 h of culture, cell distribution was relatively uniform, upregulated expression of NCAD and FAK (Figures 10
and only small cell aggregates were observed (Figure 7). and 11), suggesting structure-mediated cell condensation
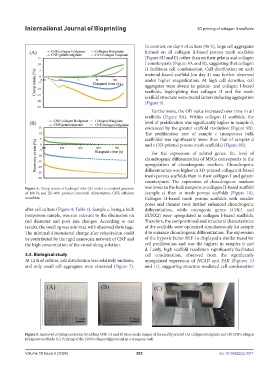
Figure 5. Improved printing resolution by adding CNF. (A and B) Macroscale images of the readily printed (A) collagen II/alginate and (B) CNF/collagen
II/alginate scaffolds. (C) Printing of the CNF/collagen/alginate ink in a cryogenic well.
Volume 10 Issue 5 (2024) 282 doi: 10.36922/ijb.3371